How Myokines Could Revolutionize Type 2 Diabetes Treatment
Discover how myokines, signaling molecules produced by skeletal muscle, could be a game-changer in the fight against type 2 diabetes. Learn about their role in regulating glucose metabolism, improving insulin sensitivity, and enhancing pancreatic beta cell function.
DR T S DIDWAL MD
9/28/20249 min read
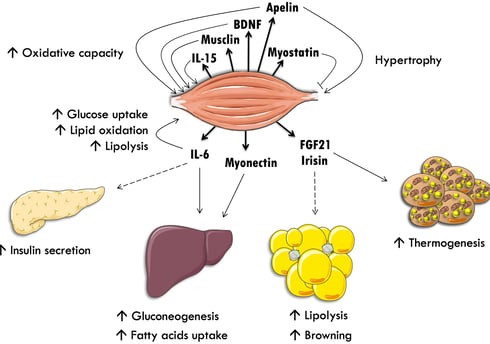
Myokines, secreted by skeletal muscle, are emerging as powerful regulators of metabolism and potential therapeutic targets for type 2 diabetes (T2D). According to research published in the International Journal of Molecular Sciences, these signaling molecules play a crucial role in glucose homeostasis, influencing insulin sensitivity, pancreatic beta cell function, and adipose tissue metabolism. Key myokines, such as irisin, FGF21, BAIBA, and SPARC, have been shown to have beneficial effects on T2D. By targeting myokine production or directly administering myokine-based therapies, researchers hope to develop innovative treatments that can improve the lives of millions affected by this chronic condition.
Key points
Myokines are signaling molecules produced by skeletal muscle.
They play a crucial role in regulating metabolism and combating type 2 diabetes (T2D).
Myokines influence insulin sensitivity, pancreatic beta cell function, and adipose tissue metabolism.
Key myokines include irisin, FGF21, BAIBA, and SPARC.
These myokines have beneficial effects on T2D, such as improving glucose uptake, promoting beta cell function, and enhancing insulin sensitivity.
Targeting myokine production or directly administering myokine-based therapies offers promising therapeutic potential for T2D.
Further research is needed to fully understand the mechanisms of myokine action and to develop effective myokine-based treatments for T2D.
The Mighty Myokines: How Muscle-Derived Molecules Could Revolutionize Type 2 Diabetes Treatment
Type 2 diabetes (T2D) has reached epidemic proportions worldwide, with over 500 million people affected and numbers projected to increase dramatically in the coming decades. This metabolic disorder is characterized by insulin resistance in peripheral tissues like skeletal muscle, as well as dysfunction of insulin-producing beta cells in the pancreas. While current treatments focus on lifestyle modifications and drugs that increase insulin sensitivity or production, many patients still struggle to maintain healthy blood glucose levels long-term.
Enter myokines - a fascinating group of molecules produced and secreted by skeletal muscle that are opening up exciting new avenues for diabetes research and treatment. In this post, we'll explore how these muscle-derived messengers could potentially revolutionize our approach to managing and even preventing T2D. We'll look at how myokines impact insulin sensitivity, glucose metabolism, and pancreatic function, as well as their broader effects on whole-body metabolism and health.
What Are Myokines?
Myokines are cytokines and peptides produced and released by muscle fibers. They act as chemical messengers, allowing skeletal muscle to communicate with other organs and tissues throughout the body. Researchers have identified over 3000 potential myokines in humans and rodents, with over 100 having confirmed functions.
Myokines can have autocrine (acting on the muscle itself), paracrine (acting on nearby cells), and endocrine (acting on distant organs) effects. They influence a wide range of physiological processes, including metabolism, inflammation, muscle growth, and tissue repair.
Importantly, many myokines are released in response to muscle contraction during exercise. This helps explain some of the wide-ranging health benefits of physical activity, as these muscle-derived molecules can improve insulin sensitivity, glucose uptake, fat oxidation, and more.
Key Myokines Involved in Glucose Metabolism and Insulin Sensitivity
Let's look at some of the most important myokines that have been implicated in glucose homeostasis and the development or prevention of T2D:
Irisin
Irisin is one of the most widely studied myokines in relation to metabolism. It's produced when the FNDC5 protein is cleaved and released into circulation during exercise. Irisin has several beneficial effects:
Increases glucose uptake in muscle cells
Improves insulin sensitivity
promotes the "browning" of white adipose tissue, increasing energy expenditure
Enhances beta cell function and insulin secretion
Protects beta cells from apoptosis
Notably, circulating irisin levels are reduced in T2D patients. This makes it an attractive target for therapeutic interventions.
FGF21 (Fibroblast Growth Factor 21)
FGF21 is another exercise-induced myokine with multiple metabolic benefits:
Lowers fasting glucose, triglycerides, and insulin levels
Increases insulin sensitivity
Promotes fat oxidation and energy expenditure
Protects against muscle atrophy and inflammation
FGF21 analogues have already advanced to clinical trials for T2D and obesity, showing promise in improving insulin sensitivity and lipid profiles.
SPARC (Secreted Protein Acidic and Rich in Cysteine)
SPARC is an exercise-responsive myokine that plays a role in glucose metabolism:
Activates AMPK signaling in muscle, improving glucose uptake
Improves glucose tolerance in obese mice
Promotes muscle development and regeneration
BAIBA (β-aminoisobutyric acid)
BAIBA is a small molecule myokine with several metabolic effects:
Improves insulin sensitivity
Increases fatty acid oxidation
Promotes the browning of white adipose tissue
Protects against diet-induced obesity and insulin resistance
IL-15 (Interleukin-15)
IL-15 is a myokine that increases after exercise and has several beneficial effects:
Promotes oxidative metabolism in muscle
Increases glucose uptake by enhancing GLUT4 expression
Protects against diet-induced obesity and insulin resistance
May have anti-aging effects on muscle
Myonectin (CTRP15)
Myonectin is involved in regulating glucose and fatty acid metabolism:
Levels increase after feeding, especially with glucose or lipids
May act as a compensatory mechanism in insulin resistance
Improves glucose tolerance and increases fatty acid oxidation
Brain-Derived Neurotrophic Factor (BDNF)
While primarily known for its roles in the nervous system, BDNF is also produced by skeletal muscle:
Promotes mitochondrial function and fatty acid oxidation
Regulates glucose metabolism in muscle
Skeletal muscle-specific BDNF knockout mice show impaired glucose utilization and insulin resistance
Myokines with Complex or Controversial Effects
Not all myokines have straightforward beneficial effects on metabolism. Some have more complex or even potentially negative impacts:
IL-6 (Interleukin-6)
IL-6 is one of the most abundantly produced myokines during exercise, but its role in metabolism is controversial:
Some studies show it improves glucose uptake and insulin sensitivity
Other research links elevated IL-6 levels to insulin resistance and inflammation
The timing and context of IL-6 release may be crucial in determining its effects
Myostatin
Myostatin is a myokine that acts as a negative regulator of muscle growth:
Inhibits muscle growth and differentiation
Myostatin deficiency or inhibition can improve insulin sensitivity and protect against obesity
However, myostatin may also play a role in regulating whole-body metabolism
These examples highlight the complexity of myokine signaling and the need for further research to fully understand their roles in health and disease.
Myokine-Mediated Muscle-to-Pancreas Communication
One of the most exciting areas of myokine research is their potential to directly influence pancreatic beta cell function. This muscle-to-pancreas cross-talk could have significant implications for T2D treatment:
Irisin: In addition to its effects on muscle and fat tissue, irisin has been shown to improve beta cell proliferation, increase insulin production, and protect beta cells from apoptosis.
Fractalkine (CX3CL1): This myokine can trigger insulin secretion by increasing intracellular calcium in beta cells. Chronic administration of a fractalkine analog in rodent models improved glucose tolerance and beta cell function.
Follistatin: While primarily known for its role in muscle growth, follistatin can also influence pancreatic function. It may protect beta cells from apoptosis and induce their proliferation.
CXCL10: This myokine, which is increased in insulin-resistant muscle, may have negative effects on beta cells, potentially inducing apoptosis.
These findings suggest that targeting muscle-derived factors could be a novel approach to preserving or enhancing beta cell function in T2D.
Myokines and Whole-Body Metabolism
The influence of myokines extends beyond muscle and the pancreas, affecting multiple organs involved in metabolism:
Adipose Tissue: Many myokines, including irisin, IL-6, and BAIBA, can influence adipose tissue metabolism. They may promote the browning of white fat, increase lipolysis, and improve insulin sensitivity in adipocytes.
Liver: Myokines like FGF21 and IL-6 can affect hepatic glucose production and lipid metabolism.
Brain: Some myokines, such as BDNF and irisin, may cross the blood-brain barrier and influence central regulation of metabolism and energy balance.
Bone: Myokines like irisin and IL-15 can affect bone metabolism, potentially influencing whole-body energy homeostasis.
This widespread influence highlights the potential of myokine-based therapies to address multiple aspects of metabolic dysfunction simultaneously.
Therapeutic Potential of Myokines in T2D
The diverse and beneficial effects of many myokines make them attractive targets for T2D treatment. Several approaches are being explored:
Direct Administration: Recombinant forms of myokines like irisin or FGF21 could be administered as drugs. FGF21 analogues have already shown promise in clinical trials, improving lipid profiles and potentially enhancing insulin sensitivity.
Stimulating Endogenous Production: Developing drugs that increase the body's own production of beneficial myokines could be another approach. This might involve targeting the pathways that regulate myokine expression and release.
Exercise Mimetics: Compounds that mimic the effects of exercise on myokine production could potentially provide some of the metabolic benefits of physical activity.
Combination Therapies: Given the complex interplay between different myokines, combination approaches targeting multiple factors simultaneously might be most effective.
Personalized Medicine: As we learn more about individual variations in myokine levels and responses, it may be possible to tailor treatments based on a patient's specific myokine profile.
Challenges and Future Directions
While the potential of myokines in T2D treatment is exciting, several challenges need to be addressed:
Translation to Humans: Many myokine studies have been conducted in animal models. More research is needed to confirm their effects in humans and determine optimal dosing and delivery methods.
Complex Interactions: Myokines don't act in isolation but as part of a complex network of signaling molecules. Understanding these interactions will be crucial for developing effective therapies.
Timing and Context: The effects of some myokines may depend on the timing of their release and the overall metabolic state. Determining the optimal conditions for intervention will be important.
Potential Side Effects: As with any biological signaling molecule, myokines may have unintended effects on other tissues or processes. Careful evaluation of safety will be essential.
Delivery and Stability: Many myokines are proteins that may be difficult to deliver effectively or may have short half-lives in the body. Developing stable, long-acting forms will be a key challenge.
Conclusion
Myokines represent a fascinating and promising area of research in the fight against type 2 diabetes. These muscle-derived molecules offer a new perspective on how skeletal muscle influences whole-body metabolism and glucose homeostasis. By improving insulin sensitivity, enhancing beta cell function, and positively affecting multiple organs involved in metabolism, myokines could potentially address many of the underlying factors contributing to T2D.
While significant challenges remain in translating this research into effective treatments, the potential rewards are substantial. Myokine-based therapies could offer more targeted and comprehensive approaches to managing T2D, potentially with fewer side effects than some current treatments.
Moreover, research into myokines underscores the importance of muscle health and physical activity in maintaining metabolic health. It provides a molecular explanation for many of the benefits of exercise and highlights the active role of skeletal muscle as an endocrine organ.
As we continue to unravel the complex world of myokines, we may be opening the door to a new era in diabetes treatment - one that harnesses the body's own molecular messengers to restore metabolic balance and improve health outcomes for millions of people worldwide.
Faqs
What are myokines?
Myokines are signaling molecules produced and secreted by skeletal muscle cells. They play a crucial role in regulating various physiological processes, including metabolism, inflammation, and muscle growth.
How do myokines relate to type 2 diabetes?
Myokines have been shown to have significant effects on glucose metabolism, insulin sensitivity, and pancreatic beta cell function. They can help improve insulin resistance, increase glucose uptake in muscle cells, and protect beta cells from damage.
What are some key myokines involved in diabetes?
Irisin: Known for its ability to increase glucose uptake and promote the browning of white adipose tissue.
FGF21: A myokine involved in regulating glucose and lipid metabolism.
BAIBA: A small molecule with potent effects on insulin sensitivity and fatty acid oxidation.
SPARC: Plays a role in glucose metabolism and muscle development.
How can myokines be used to treat type 2 diabetes?
Researchers are investigating various approaches to harness the therapeutic potential of myokines, including:
Direct administration: Using recombinant myokines as drugs.
Stimulating endogenous production: Developing drugs that increase the body's natural production of myokines.
Exercise mimetics: Compounds that mimic the effects of exercise on myokine production.
Are there any risks associated with myokine-based therapies?
While myokines show promise, more research is needed to fully understand their potential side effects and long-term consequences.
How can I naturally increase myokine production?
Regular physical activity, especially resistance training, is one of the most effective ways to increase myokine production. A healthy diet and adequate sleep can also contribute to optimal myokine levels.
Can myokines help prevent type 2 diabetes?
Yes, myokines can play a role in preventing type 2 diabetes by improving insulin sensitivity and regulating glucose metabolism.
Are there any ongoing clinical trials for myokine-based therapies?
Yes, there are several ongoing clinical trials investigating the use of myokines, such as FGF21, for the treatment of type 2 diabetes.
Can myokines help with other health conditions besides diabetes?
Myokines have been implicated in various other health conditions, including obesity, cardiovascular disease, and muscle wasting. Further research is needed to explore their therapeutic potential in these areas.
Related Article
Exercise and Myokine Production: The Key to Metabolic Boost ,Weight loss and DIabetes control
Journal Reference
Balakrishnan, R., & Thurmond, D. C. (2022). Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance. International Journal of Molecular Sciences, 23(9). https://doi.org/10.3390/ijms23094636
Image credit: https://www.frontiersin.org/files/Articles/467932/fphys-11-00091-HTML/image_m/fphys-11-00091-g001.jpg
Disclaimer
The information on this website is for educational and informational purposes only and is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or treatment, and before undertaking a new healthcare regimen, and never disregard professional medical advice or delay in seeking it because of something you have read on this website.
