mRNA Therapeutics: Breakthrough Treatments for Inherited Neurological Conditions
"Discover how mRNA therapeutics are offering groundbreaking treatments for inherited neurological conditions like Spinal Muscular Atrophy (SMA) and Huntington's Disease, bringing new hope to patients with genetic disorders."
9/29/20249 min read
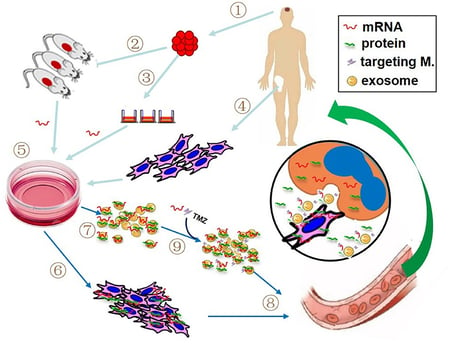
mRNA technology, initially recognized for its role in COVID-19 vaccines, holds great promise for treating inherited neurological diseases caused by single-gene mutations, such as Spinal Muscular Atrophy (SMA) and Huntington's Disease. These conditions often result from missing or malfunctioning proteins, leading to severe health complications. According to research published in the journal Brain, while traditional treatments focus on managing symptoms, mRNA therapeutics offer a groundbreaking approach by instructing cells to produce the correct protein, bypassing the need to modify the faulty gene. Scientists design mRNA sequences that code for healthy versions of the missing or defective protein, package them, and administer them to patients. Once inside the cells, the mRNA instructs protein production, potentially correcting the disorder's effects. This method is flexible, can target multiple diseases, and doesn't alter DNA, reducing the risk of long-term genetic changes. Additionally, the temporary nature of mRNA allows for better control over treatment. However, challenges such as delivering mRNA to the brain, ensuring its stability, and preventing immune responses remain. Despite these hurdles, ongoing research and clinical trials for conditions like SMA, Parkinson's, and ALS offer hope for a new era in neurological treatment.
Key points
Revolutionary Potential: mRNA technology offers a new approach to treating inherited neurological diseases by helping cells produce missing or defective proteins.
Targeting Monogenic Disorders: It is particularly promising for monogenic loss-of-function diseases like Spinal Muscular Atrophy (SMA) and Huntington's Disease, caused by mutations in a single gene.
Flexible and Precise: mRNA therapeutics can be tailored to address different mutations, offering precision and flexibility in treatment.
Temporary and Safe: mRNA therapy does not integrate into the DNA, reducing the risk of long-term genetic changes, and its effects are temporary, allowing better control.
Challenges to Overcome: Key hurdles include delivering mRNA to the brain, ensuring stability, and minimizing immune responses to repeated treatments.
Ongoing Research: Clinical trials are already exploring mRNA therapies for conditions like SMA, Parkinson's Disease, and ALS, showing early promise.
New Era in Medicine: With continued advances, mRNA therapeutics could transform the treatment landscape for inherited neurological diseases, offering more effective and personalized solutions.
The Promise of mRNA Therapeutics for Inherited Neurological Diseases: A New Frontier in Medicine
In the ever-evolving landscape of medical research, few developments have captured the imagination of scientists and the public alike as much as mRNA technology. While its recent claim to fame has been in the realm of COVID-19 vaccines, the potential applications of mRNA extend far beyond infectious diseases. One of the most exciting frontiers for this groundbreaking technology is in the treatment of inherited neurological disorders. In this comprehensive blog post, we'll explore the promising world of mRNA therapeutics and its potential to revolutionize the treatment of monogenic loss-of-function neurological diseases.
Understanding Neurological Monogenic Loss-of-Function Diseases
Before diving into the potential of mRNA therapeutics, it's crucial to understand the challenges posed by neurological monogenic loss-of-function diseases. These are inherited disorders resulting from mutations in a single gene that lead to a decrease or complete loss of the normal function of the encoded protein. Such conditions can have devastating effects on the nervous system, often leading to severe disabilities or shortened lifespans.
Examples of these disorders include:
Spinal Muscular Atrophy (SMA)
Duchenne Muscular Dystrophy (DMD)
Huntington's Disease
Friedreich's Ataxia
Some forms of Parkinson's Disease
Historically, these conditions have been notoriously difficult to treat. Traditional approaches often focus on managing symptoms rather than addressing the root cause of the disease. This is where mRNA therapeutics enter the picture, offering a potentially game-changing approach to treatment.
The Rise of mRNA Technology
The concept of using mRNA as a therapeutic tool has been around for decades, but it wasn't until recently that the technology matured enough for practical applications. The breakthrough success of mRNA-based COVID-19 vaccines has dramatically accelerated interest and investment in this field, opening up new possibilities for treating a wide range of diseases.
mRNA, or messenger RNA, acts as a blueprint for protein production in our cells. By introducing therapeutic mRNA into cells, we can potentially instruct them to produce specific proteins that are missing or defective in certain diseases. This approach offers several advantages over traditional gene therapy:
Mutation-agnostic approach: mRNA therapy can potentially address virtually any monogenic loss-of-function disease, regardless of the specific mutation involved.
Simplified delivery: Unlike DNA-based gene therapies, mRNA only needs to reach the cell's cytoplasm, not the nucleus, reducing the complexity of delivery and minimizing risks associated with DNA integration.
Transient effect: The effects of mRNA therapy are temporary, which can be advantageous in terms of safety and control.
Cost-effectiveness: mRNA production is relatively straightforward and can be scaled up efficiently, potentially leading to more affordable treatments.
mRNA Therapeutics for Neurological Diseases: How It Works
The basic principle behind mRNA therapeutics for neurological diseases is elegant in its simplicity. Here's a step-by-step breakdown of how it works:
Design: Scientists design an mRNA sequence that codes for the healthy version of the protein that is defective or missing in the disease.
Packaging: The mRNA is packaged into a delivery vehicle, often lipid nanoparticles, which protect the mRNA and help it enter cells.
Administration: The packaged mRNA is administered to the patient, typically through injection or infusion.
Cellular uptake: The mRNA enters the target cells in the nervous system.
Protein production: Once inside the cell, the mRNA is used as a template by the cell's own protein-making machinery to produce the healthy protein.
Therapeutic effect: The newly produced protein carries out its normal function, potentially correcting or mitigating the effects of the disease.
This approach bypasses the need to correct the underlying genetic mutation, instead providing the instructions for the cell to produce the correct protein directly.
Advantages of mRNA Therapeutics for Neurological Diseases
mRNA therapeutics offer several unique advantages that make them particularly promising for treating neurological disorders:
Precision: mRNA can be designed to produce exactly the protein needed, with the correct post-translational modifications.
Flexibility: The mRNA sequence can be easily modified to optimize protein production or to address different mutations of the same gene.
Safety: Since mRNA doesn't integrate into the genome, it poses a lower risk of unintended long-term genetic changes compared to DNA-based gene therapies.
Dosage control: The transient nature of mRNA allows for better control over the duration and level of protein production.
Repeat administration: Unlike some gene therapies that can only be administered once due to immune responses, mRNA treatments can potentially be given repeatedly.
Current Challenges and Ongoing Research
While the potential of mRNA therapeutics is enormous, several challenges need to be addressed to fully realize this potential in treating neurological diseases:
Delivery to the CNS: One of the biggest hurdles is effectively delivering mRNA across the blood-brain barrier to reach the central nervous system.
Stability: mRNA is inherently unstable and can be quickly degraded in the body. Improving its stability without compromising function is an active area of research.
Immune responses: Repeated administration of mRNA can potentially trigger immune responses, which could reduce efficacy or cause side effects.
Dosing and duration: Determining the optimal dosing regimen and treatment duration for chronic conditions is crucial.
Manufacturing and storage: Ensuring consistent, high-quality production and developing suitable storage conditions for mRNA therapeutics are ongoing challenges.
Researchers are actively working on these issues, with promising advances being made in areas such as:
Novel delivery systems, including engineered nanoparticles and exosomes
Chemical modifications to improve mRNA stability and reduce immunogenicity
Targeted delivery strategies to enhance specificity for neuronal cells
Optimization of mRNA sequences for improved protein expression
Promising Applications and Ongoing Clinical Trials
Several mRNA-based therapies for neurological disorders are currently in various stages of development and clinical testing. Some notable examples include:
Spinal Muscular Atrophy (SMA): Researchers are exploring mRNA therapy to produce the SMN protein, which is deficient in SMA patients.
Huntington's Disease: mRNA approaches are being investigated to reduce the production of the mutant huntingtin protein or to produce neuroprotective factors.
Friedreich's Ataxia: mRNA therapy aimed at restoring frataxin protein levels is under development.
Parkinson's Disease: While not always monogenic, some forms of Parkinson's are being targeted with mRNA therapies to produce neuroprotective factors or correct specific genetic defects.
Amyotrophic Lateral Sclerosis (ALS): mRNA approaches are being explored for various genetic forms of ALS.
These are just a few examples of the wide range of neurological conditions that could potentially benefit from mRNA therapeutics.
The Future of mRNA Therapeutics in Neurology
As research in this field progresses, we can expect to see several exciting developments:
Personalized treatments: The flexibility of mRNA technology could allow for highly personalized therapies tailored to an individual's specific genetic mutation.
Combination therapies: mRNA treatments could be combined with other therapeutic approaches, such as small molecules or antibodies, for enhanced efficacy.
Expanded applications: As delivery methods improve, mRNA therapeutics could be applied to a broader range of neurological conditions, including more complex polygenic disorders.
Preventive strategies: For diseases with known genetic causes, mRNA therapies could potentially be used preventively in high-risk individuals before symptoms appear.
Improved diagnostics: The development of mRNA therapeutics is likely to drive advances in genetic testing and biomarker discovery, leading to earlier and more accurate diagnosis of neurological disorders
.
Ethical and Societal Implications
As with any groundbreaking medical technology, the advancement of mRNA therapeutics for neurological diseases raises important ethical and societal questions:
Access and affordability: How can we ensure that these potentially life-changing treatments are accessible to all who need them?
Long-term effects: What are the potential long-term consequences of modifying protein production in the nervous system?
Genetic screening: As treatments become available, how will this impact genetic screening practices and family planning decisions?
Regulation and approval: How should regulatory frameworks evolve to appropriately evaluate the safety and efficacy of these novel therapies?
Resource allocation: How will healthcare systems prioritize the development and implementation of these treatments alongside other medical needs?
Addressing these questions will require ongoing dialogue between scientists, healthcare providers, policymakers, ethicists, and patient advocacy groups.
Conclusion: A New Era in Neurological Treatment
The emergence of mRNA therapeutics represents a potential paradigm shift in the treatment of inherited neurological diseases. By providing a flexible, precise, and potentially safer alternative to traditional gene therapies, mRNA technology offers new hope for patients and families affected by these often devastating conditions.
While challenges remain, the rapid pace of research and the lessons learned from the successful development of mRNA vaccines suggest that we are on the cusp of a new era in neurological treatment. As we continue to unlock the full potential of mRNA therapeutics, we may soon see transformative treatments that can significantly improve the lives of those affected by monogenic loss-of-function neurological diseases.
The journey from concept to clinical reality is never straightforward, but the promise of mRNA therapeutics in neurology is too great to ignore. As researchers, clinicians, and patients alike look to the future, there is renewed optimism that we may finally have the tools to address some of the most challenging neurological disorders head-on. The mRNA revolution in neurology is just beginning, and its full impact is yet to be realized
Faqs.
1. What are mRNA therapeutics?
mRNA therapeutics involve using messenger RNA (mRNA) to instruct cells to produce specific proteins. Inherited neurological diseases caused by genetic mutations often result in missing or defective proteins. mRNA therapy can provide a blueprint for the cell to produce the correct protein, potentially treating or managing the disease.
2. How does mRNA therapy differ from traditional gene therapy?
Unlike traditional gene therapy, which attempts to fix or replace faulty genes, mRNA therapy doesn’t alter the patient’s DNA. It instead provides the instructions for cells to produce a functional protein directly. mRNA therapy is generally safer since it doesn’t risk long-term genetic changes.
3. What neurological diseases can mRNA therapy treat?
mRNA therapy is being explored for several inherited neurological conditions, such as Spinal Muscular Atrophy (SMA), Huntington’s Disease, Friedreich’s Ataxia, and some forms of Parkinson’s Disease. Clinical trials are ongoing for these and other conditions.
4. How is mRNA therapy administered?
The therapeutic mRNA is typically delivered through injection or infusion. The mRNA is packaged in lipid nanoparticles or similar delivery systems that help it enter the body’s cells, where it instructs protein production.
5. What are the benefits of mRNA therapy?
mRNA therapy offers precision, flexibility, and safety. It allows the body to produce exactly the protein needed without altering DNA, offering a more controlled and repeatable treatment. It can also be adapted for various diseases by modifying the mRNA sequence.
6. What are the challenges of using mRNA for neurological diseases?
Key challenges include delivering mRNA to the brain, crossing the blood-brain barrier, maintaining mRNA stability in the body, and preventing immune responses that may reduce the treatment’s effectiveness or cause side effects.
7. Are there any mRNA therapies for neurological diseases available now?
Currently, mRNA therapies for neurological diseases are in the research and clinical trial stages. While mRNA-based vaccines for infectious diseases like COVID-19 are approved, neurological applications are still undergoing extensive testing to ensure safety and efficacy.
8. Is mRNA therapy a permanent solution?
No, mRNA therapy provides a temporary effect since mRNA is naturally degraded by the body. This means that treatment may need to be administered multiple times, allowing for greater control over dosing and minimizing long-term risks.
9. What does the future hold for mRNA therapeutics in neurology?
Ongoing research is optimistic about mRNA therapy’s potential to treat more complex neurological conditions and possibly combine it with other therapies for enhanced efficacy. It could also pave the way for personalized treatments tailored to individual genetic profiles.
Related Article
The Science Behind Daydreaming: Default Mode Network and Creative Thinking
Journal Reference
Edoardo Monfrini, Giacomo Baso, Dario Ronchi, Megi Meneri, Delia Gagliardi, Lorenzo Quetti, Federico Verde, Nicola Ticozzi, Antonia Ratti, Alessio Di Fonzo, Giacomo P Comi, Linda Ottoboni, Stefania Corti, Unleashing the potential of mRNA therapeutics for inherited neurological diseases, Brain, Volume 147, Issue 9, September 2024, Pages 2934–2945, https://doi.org/10.1093/brain/awae135
Image credit: https://www.frontiersin.org/files/Articles/494123/fonc-09-01208-HTML/image_m/fonc-09-01208-g002.jpg
Disclaimer
The information on this website is for informational purposes only and is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health care provider with any questions you may have regarding a medical condition or treatment. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
