Preventing Muscle Wasting: Combating Fat Infiltration in Aging Muscles
Discover effective strategies to combat muscle loss and fat infiltration in aging muscles. Learn about the benefits of regular exercise, proper nutrition, and lifestyle modifications for maintaining muscle strength and overall health as you age.
DR T S DIDWAL MD
10/12/202411 min read
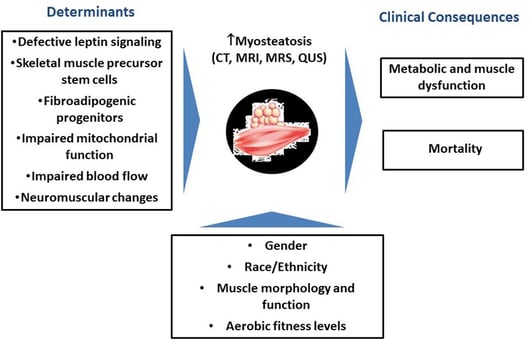
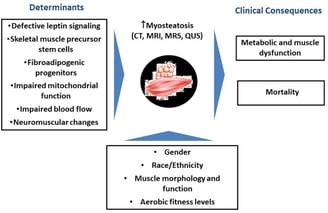
The infiltration of fat into skeletal muscles, known as myosteatosis, is a common consequence of aging. This phenomenon, which can lead to reduced muscle strength, altered muscle architecture, and impaired muscle contraction, is associated with various metabolic disorders. Recent research published in Scientific Data has identified several genes involved in this process and highlighted the importance of fibro/adipogenic progenitors (FAPs) in fat cell formation within muscle tissue. Understanding the mechanisms of myosteatosis could lead to new strategies for maintaining muscle health and function in older adults.
Key points
Myosteatosis: The accumulation of intramuscular fat (IMF) in skeletal muscles.
Aging and Myosteatosis: Myosteatosis is a standard feature of aging and is associated with the decline in muscle function.
Molecular Mechanisms: Genes like VIM, AGT, ADIPOQ, FABP4, PPARG, CPT1A, and SCD are upregulated in aging muscles with increased fat infiltration.
Cross-Species Insights: Similarities between human and pig muscles in terms of fat infiltration highlight the evolutionary conservation of certain biological processes.
Cellular Players: FAPs, fibroblasts, endothelial cells, immune cells, MuSCs, pericytes, adipocytes, and myeloid-derived cells play roles in myosteatosis.
FAPs and Fat Infiltration: FAPs can give rise to preadipocytes, contributing to intramuscular fat deposition.
Future Directions: Further research is needed to explore the functional heterogeneity of muscle fibers, epigenetic modifications, environmental factors, in vitro models, and cell-based therapies related to myosteatosis.
Unraveling the Connection Between Aging and Fat Infiltration in Skeletal Muscle
As we age, our bodies undergo numerous changes, some more visible than others. One significant but often overlooked change is the infiltration of fat into our skeletal muscles, a phenomenon known as myosteatosis. This process, which involves the accumulation of intramuscular fat (IMF), has far-reaching implications for our health, mobility, and quality of life as we grow older. In this blog post, we'll dive deep into the fascinating world of myosteatosis, exploring its relationship with aging and the cutting-edge research that's helping us understand this complex biological process.
The Aging Muscle: More Than Meets the Eye
When we think about aging muscles, we often focus on the visible signs: reduced muscle mass, decreased strength, and slower recovery from physical activity. However, beneath the surface, a more insidious change is taking place. As we age, our skeletal muscles begin to accumulate fat, a process that can have profound effects on muscle function and overall health.
This fat infiltration, or myosteatosis, is now recognized as a standard feature of aging. It's directly related to the decline in muscle function that many older adults experience. But why does this happen, and what are the consequences?
Myosteatosis: The Silent Invader
Myosteatosis refers to the pathological fat accumulation in skeletal muscle. Unlike the subcutaneous fat that we can pinch with our fingers, intramuscular fat is deposited within and between muscle fibers. This fat infiltration can lead to:
Reduced muscle strength
Altered muscle architecture
Impaired muscle contraction
Decreased muscle capacity
These changes contribute to the age-related decline in physical function, often referred to as sarcopenia. But myosteatosis isn't just a concern for the elderly. It's also associated with various metabolic disorders, including diabetes and obesity.
The Molecular Fingerprints of Aging and Fat Infiltration
Recent advances in genetic research have allowed scientists to peek into the molecular world of aging muscles. By analyzing RNA sequencing data from human and animal studies, researchers have identified several key genes that are upregulated in aging muscles with increased fat infiltration.
Some of the notable genes include:
VIM (Vimentin)
AGT (Angiotensinogen)
ADIPOQ (Adiponectin)
FABP4 (Fatty Acid Binding Protein 4)
PPARG (Peroxisome Proliferator Activated Receptor Gamma)
CPT1A (Carnitine Palmitoyltransferase 1A)
SCD (Stearoyl-CoA Desaturase)
These genes play crucial roles in adipogenesis (fat cell formation) and lipid metabolism. Their increased expression in aging muscles provides strong evidence for the link between aging and fat infiltration.
From Humans to Pigs: Cross-Species Insights
While human studies are invaluable, animal models provide additional insights into the mechanisms of myosteatosis. Interestingly, researchers have found striking similarities between humans and pigs in terms of fat infiltration in skeletal muscles.
The Laiwu pig, a Chinese breed known for its high intramuscular fat content, has emerged as an excellent model for studying myosteatosis. By comparing the genetic profiles of aged human muscles and high-IMF content pig muscles, scientists have identified conserved gene programs associated with fat infiltration across species.
This cross-species approach not only validates the relevance of animal models but also highlights the evolutionary conservation of certain biological processes related to aging and fat metabolism.
The Cellular Players in Myosteatosis
Understanding myosteatosis requires a deep dive into the cellular composition of skeletal muscle. Thanks to advanced techniques like single-nucleus RNA sequencing (snRNA-seq), researchers can now identify and characterize the various cell types present in muscle tissue with unprecedented resolution.
In aged human muscle, several key cell populations have been identified:
Myofibers (muscle cells)
Fibro/adipogenic progenitors (FAPs) and fibroblasts
Endothelial cells
Immune cells
Muscle stem cells (MuSCs)
Pericytes
Interestingly, studies in pigs have revealed additional cell types, including adipocytes and myeloid-derived cells. These differences highlight the complexity of muscle tissue and the potential species-specific aspects of fat infiltration.
FAPs: The Key Players in Fat Infiltration
Among the various cell types present in muscle tissue, fibro/adipogenic progenitors (FAPs) have emerged as central players in the process of fat infiltration. These cells, which express platelet-derived growth factor receptor α (PDGFRα), have the potential to differentiate into both fat cells and fibrous tissue.
Recent research has shown that FAPs can give rise to a specific subpopulation of preadipocytes characterized by the expression of PDE4D and PDE7B genes. This finding suggests a potential pathway from FAPs to mature fat cells within the muscle tissue.
The identification of these PDE4D+/PDE7B+ preadipocytes in both human and pig muscle samples underscores their importance in the process of intramuscular fat deposition. This discovery opens up new avenues for potential therapeutic interventions aimed at controlling fat infiltration in aging muscles.
The Complex Web of Cellular Interactions
Muscle tissue is far from a simple collection of muscle fibers. It's a complex ecosystem where various cell types interact and influence each other's behavior. Using sophisticated computational techniques, researchers have begun to unravel the intricate web of cell-cell communications within aging muscle tissue.
One intriguing finding is the potential role of specific collagen proteins in mediating interactions between FAPs and other cell types with adipogenic potential. The COL4A2 and COL6A3 pathways, in particular, have been implicated in these cellular dialogues.
These interactions highlight the dynamic nature of muscle tissue and suggest that fat infiltration is not simply the result of a single cell type gone awry, but rather a complex process involving multiple cellular players.
Mechanisms Contributing to Myosteatosis
Myosteatosis, the infiltration of fat into skeletal muscle, is a complex process involving multiple cellular and molecular mechanisms. Here's a deeper look at some of the key factors and how they contribute to this phenomenon:
1. Age-Related Cellular Changes
Satellite Cell Dysfunction
Mechanism: With age, the number and function of satellite cells (muscle stem cells) decline.
Impact: Reduced muscle regeneration capacity, leading to gradual replacement of muscle tissue with fat.
Mitochondrial Dysfunction
Mechanism: Aging muscles show decreased mitochondrial number and function.
Impact: Reduced energy production and increased oxidative stress, promoting muscle atrophy and fat accumulation.
2. Hormonal Changes
Decreased Growth Hormone and IGF-1
Mechanism: Age-related decline in growth hormone and insulin-like growth factor 1 (IGF-1) levels.
Impact: Reduced muscle protein synthesis and increased fat deposition.
Insulin Resistance
Mechanism: Age and lifestyle factors can lead to decreased insulin sensitivity.
Impact: Impaired glucose uptake by muscle cells, promoting fat storage instead of energy utilization.
3. Chronic Low-Grade Inflammation
Increased Pro-Inflammatory Cytokines
Mechanism: Aging is associated with increased levels of inflammatory markers like TNF-α and IL-6.
Impact: Chronic inflammation can lead to muscle protein breakdown and promote adipogenesis.
4. Alterations in Muscle Fiber Types
Shift from Type II to Type I Fibers
Mechanism: Age-related preferential loss of fast-twitch (Type II) muscle fibers.
Impact: Type I fibers are more susceptible to fat infiltration, altering muscle composition.
5. Changes in Adipokine Signaling
Altered Adiponectin and Leptin Levels
Mechanism: Dysregulation of fat-derived hormones like adiponectin and leptin.
Impact: These changes can affect muscle metabolism and promote fat accumulation.
6. Vascular Changes
Reduced Muscle Capillarization
Mechanism: Decrease in the number of capillaries supplying muscle fibers.
Impact: Reduced nutrient delivery and waste removal, potentially promoting fat accumulation.
7. Epigenetic Modifications
DNA Methylation and Histone Modifications
Mechanism: Age-related changes in epigenetic markers affecting gene expression.
Impact: Altered expression of genes involved in muscle maintenance and fat metabolism.
8. Dysregulation of Fibro/Adipogenic Progenitors (FAPs)
Increased FAP Activation
Mechanism: Age or injury can lead to increased activation of FAPs.
Impact: Greater potential for these cells to differentiate into adipocytes within muscle tissue.
9 Altered Lipid Metabolism
Impaired Fatty Acid Oxidation
Mechanism: Decreased activity of enzymes involved in fatty acid breakdown, like CPT1.
Impact: Reduced ability to use fats for energy, leading to increased storage within muscle.
Increased Intramyocellular Lipid Accumulation
Mechanism: Age-related increase in lipid droplets within muscle fibers.
Impact: Can lead to lipotoxicity and further impairment of muscle function.
From Bench to Bedside: Implications for Human Health
The insights gained from studying myosteatosis have significant implications for human health, particularly in the context of aging and age-related disorders. Understanding the mechanisms of fat infiltration could lead to new strategies for maintaining muscle health and function in older adults.
Some potential applications include:
Developing biomarkers for early detection of myosteatosis
Identifying targets for therapeutic interventions to prevent or reverse fat infiltration
Designing personalized exercise and nutrition programs to maintain muscle quality
Improving our understanding of metabolic disorders associated with muscle dysfunction
Moreover, the similarities observed between human and pig muscles in terms of fat infiltration processes validate the use of porcine models in biomedical research. This could accelerate the translation of laboratory findings into clinical applications.
The Road Ahead: Future Directions in Myosteatosis Research
While we've made significant strides in understanding myosteatosis, many questions remain unanswered. Future research directions may include:
Exploring the functional heterogeneity of muscle fibers at the single-nucleus level
Investigating the role of epigenetic modifications in fat infiltration
Studying the impact of environmental factors on myosteatosis
Developing in vitro models to study the dynamics of fat infiltration in real-time
Exploring the potential of cell-based therapies to combat muscle aging and fat infiltration
Preventing Muscle Wasting and Fat Infiltration
While the aging process and its effects on muscle tissue are complex, research suggests several strategies that may help prevent or slow down muscle wasting (sarcopenia) and fat infiltration (myosteatosis). Here are some key approaches:
1. Regular Exercise:
Resistance training: Strength exercises help maintain and build muscle mass. Aim for at least two sessions per week.
Aerobic exercise: Activities like walking, swimming, or cycling can help maintain overall muscle health and reduce fat accumulation.
2. Proper Nutrition:
Adequate protein intake: Consume enough high-quality protein (1.2-1.6 g/kg of body weight per day for older adults) to support muscle maintenance and growth.
Balanced diet: Ensure a diet rich in fruits, vegetables, whole grains, and healthy fats to provide necessary nutrients and antioxidants.
3. Vitamin D and Calcium:
Maintain adequate levels of vitamin D and calcium, which are crucial for muscle and bone health.
Consider supplementation if dietary intake is insufficient, under medical supervision.
4. Hormone Balance:
Monitor and maintain healthy hormone levels, particularly growth hormone and testosterone, which play roles in muscle maintenance.
Consult with a healthcare provider about hormone replacement therapy if appropriate.
5. Adequate Sleep:
- Aim for 7-9 hours of quality sleep per night, as sleep is crucial for muscle recovery and overall health.
6. Stress Management:
Chronic stress can contribute to muscle wasting. Engage in stress-reduction techniques like meditation, yoga, or deep breathing exercises.
7. Stay Hydrated:
Proper hydration is essential for muscle function and overall health.
8. Novel Interventions (under research):
Targeted therapies to inhibit myostatin, a protein that limits muscle growth.
nterventions aimed at regulating the activity of fibro/adipogenic progenitors (FAPs) to reduce fat infiltration.
Remember, these strategies are most effective when implemented consistently over time. It's never too late to start, but the earlier these habits are adopted, the better the outcomes tend to be. Always consult with a healthcare provider before starting any new exercise regimen or making significant changes to your diet, especially if you have pre-existing health conditions.## The Road Ahead: Future Directions in Myosteatosis Research
Conclusion: A New Frontier in Aging Research
The study of myosteatosis represents a new frontier in aging research, bridging the fields of muscle biology, metabolism, and gerontology. By unraveling the complex relationships between aging, fat infiltration, and muscle function, we're gaining invaluable insights into the aging process itself.
As we continue to peel back the layers of this biological onion, we're not just satisfying scientific curiosity. We're paving the way for innovative approaches to healthy aging, potentially improving the quality of life for millions of older adults around the world.
The journey to understand myosteatosis is far from over, but with each discovery, we're one step closer to cracking the code of muscle aging. And in doing so, we're writing a new chapter in the story of human health and longevity.
FAQs
1. What is myosteatosis? Myosteatosis is the pathological accumulation of intramuscular fat (IMF) within and between muscle fibers. It's a common feature of aging and is associated with the decline in muscle function.
2. What are the consequences of myosteatosis? Myosteatosis can lead to:
Reduced muscle strength
Altered muscle architecture
Impaired muscle contraction
Decreased muscle capacity
3. What are the molecular mechanisms involved in myosteatosis? Several genes, including VIM, AGT, ADIPOQ, FABP4, PPARG, CPT1A, and SCD, are upregulated in aging muscles with increased fat infiltration.
4. What are the cellular players in myosteatosis? Various cell types, including FAPs, fibroblasts, endothelial cells, immune cells, MuSCs, pericytes, adipocytes, and myeloid-derived cells, contribute to myosteatosis.
5. What is the role of FAPs in myosteatosis? Fibro/adipogenic progenitors (FAPs) can differentiate into both fat cells and fibrous tissue, playing a crucial role in intramuscular fat deposition.
6. Are there any potential treatments for myosteatosis? Current research is focused on understanding the underlying mechanisms of myosteatosis to develop potential therapeutic interventions. However, maintaining a healthy lifestyle, including regular exercise and a balanced diet, can help mitigate the effects of muscle aging.
7. Can myosteatosis be prevented? While it's not possible to completely prevent myosteatosis, a healthy lifestyle, including regular physical activity and a balanced diet, can help to slow its progression.
8. Is myosteatosis only a problem for older adults? While myosteatosis is more common in older adults, it can also affect younger individuals with certain metabolic disorders or sedentary lifestyles.
Glossary of Terms: Myosteatosis and Aging
Adipogenesis: The process of cell differentiation by which pre-adipocytes become adipocytes (fat cells).
Adiponectin (ADIPOQ): A protein hormone that regulates glucose levels and fatty acid breakdown.
Angiotensinogen (AGT): A protein that is converted to angiotensin I and ultimately to angiotensin II, which regulates blood pressure.
Carnitine Palmitoyltransferase 1A (CPT1A): An enzyme crucial for the beta-oxidation of long-chain fatty acids.
Endothelial cells (ECs): Cells that line the interior surface of blood vessels and lymphatic vessels.
Epigenetic modifications: Changes in gene expression that don't involve changes to the underlying DNA sequence.
Fatty Acid Binding Protein 4 (FABP4): A protein involved in fatty acid uptake, transport, and metabolism.
Fibro/adipogenic progenitors (FAPs): A type of progenitor cell in skeletal muscle that can differentiate into adipocytes or fibroblasts.
Fibroblasts: Cells that produce collagen, the structural framework for animal tissues.
Immunological cells: Cells of the immune system that protect the body against pathogens and foreign substances.
Intramuscular fat (IMF): Fat stored within the muscle tissue.
Laiwu pig: A Chinese pig breed known for its high intramuscular fat content.
Mesenchymal stem cells (MSCs): Multipotent stromal cells that can differentiate into a variety of cell types.
Muscle stem cells (MuSCs): Also known as satellite cells, these are the primary stem cells in skeletal muscle.
Myofibers: The long, cylindrical cells that form skeletal muscle tissue.
Myosteatosis: The pathological fat accumulation in skeletal muscle.
Myeloid-derived cells: Cells that originate from the bone marrow and can develop into various types of blood cells.
Pericytes: Contractile cells that wrap around the endothelial cells of capillaries and venules.
Peroxisome Proliferator Activated Receptor Gamma (PPARG): A nuclear receptor that regulates fatty acid storage and glucose metabolism.
Phosphodiesterase 4D (PDE4D): An enzyme involved in regulating intracellular levels of cyclic AMP.
Phosphodiesterase 7B (PDE7B): Another enzyme involved in the regulation of cyclic AMP.
Platelet-derived growth factor receptor α (PDGFRα): A cell surface receptor for members of the platelet-derived growth factor family.
RNA sequencing (RNA-seq): A technique used to analyze the presence and quantity of RNA in a biological sample at a given moment.
Sarcopenia: The degenerative loss of skeletal muscle mass, quality, and strength associated with aging.
Single-nucleus RNA sequencing (snRNA-seq): A method for measuring gene expression in individual nuclei, useful for studying complex tissues.
Stearoyl-CoA Desaturase (SCD): An enzyme involved in fatty acid biosynthesis.
Vimentin (VIM): A protein that makes up the intermediate filaments of the cytoskeleton in many cell types.
Related Article
Creatine Supplements to Boost Strength, Power, and Muscle Mass: A Scientific Look at its Benefits
Resistance Circuit Training: Fight Aging and enhance Strength, Balance, and Mobility
Journal Reference
Wang, L., Zhou, Y., Wang, Y., & Shan, T. (2024). Integrative cross-species analysis reveals conserved and unique signatures in fatty skeletal muscles. Scientific Data, 11(1), 1-14. https://doi.org/10.1038/s41597-024-03114-5
Wang, L., Valencak, T. G., & Shan, T. (2024). Fat infiltration in skeletal muscle: Influential triggers and regulatory mechanism. IScience, 27(3), 109221. https://doi.org/10.1016/j.isci.2024.109221
Image credit: https://www.frontiersin.org/files/Articles/549054/fphys-11-00963-HTML/image_m/fphys-11-00963-g001.jpg
Disclaimer
