Should You Be Worried About Homocysteine? What Science Says About Heart Risk
Is high homocysteine a hidden threat to your heart? Learn what the latest science says and how to lower your cardiovascular risk
DR T S DIDWAL MD
4/15/20259 min read
Homocysteine and Cardiovascular Disease: The Hidden Connection You Need to Know About
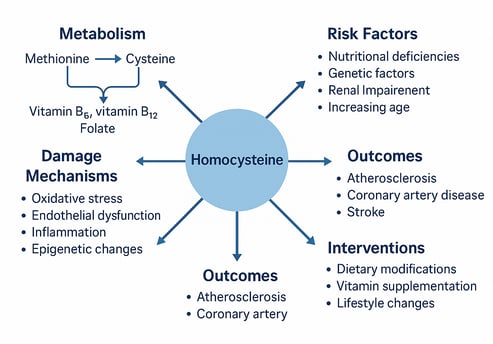
Homocysteine—ever heard of it? It’s a sulfur-containing amino acid that quietly builds up in your blood when certain B vitamins (like B6, B12, and folate) are lacking. When levels rise too high, a condition called hyperhomocysteinemia (HHcy) occurs—and that’s where things get serious for your heart.
Studies increasingly show that elevated homocysteine levels are linked to cardiovascular disease (CVD), including atherosclerosis, heart failure (especially HFpEF), and coronary syndromes. So, what’s the damage? This sneaky molecule can trigger oxidative stress, endothelial dysfunction, inflammation, and even epigenetic changes that set the stage for vascular injury.
Despite the clear association, lowering homocysteine with B-vitamin supplements hasn’t consistently reduced heart events in large trials. Why the gap? Likely due to genetic differences, baseline health, and timing of intervention.
The good news? You can lower your homocysteine naturally—with folate-rich foods, physical activity, quitting smoking, and a Mediterranean-style diet. Emerging therapies are also targeting oxidative and epigenetic pathways for a more tailored approach.
But what exactly is the relationship between homocysteine and cardiovascular health? Is it a causative factor or merely a marker? Can managing homocysteine levels effectively reduce cardiovascular risk? This comprehensive review aims to shed light on these questions by examining the latest research and exploring the mechanisms through which homocysteine affects vascular biology.
Understanding Homocysteine: The Basics
Homocysteine is a sulfur-containing amino acid that plays a crucial role in various metabolic processes. It's not obtained directly from diet but rather forms as an intermediate product during the metabolism of methionine, an essential amino acid found in protein-rich foods.
Under normal conditions, homocysteine is converted back to methionine or transformed into cysteine through processes that require adequate levels of B vitamins—particularly folate (B9), vitamin B6, and vitamin B12. When these pathways are disrupted due to nutritional deficiencies, genetic factors, or other conditions, homocysteine levels can become elevated in the bloodstream.
Hyperhomocysteinemia (HHcy) refers to abnormally high levels of homocysteine in the blood. While definitions vary, levels above 12-15 μmol/L are generally considered elevated. The severity of HHcy can be classified as:
Moderate elevation: 15-30 μmol/L
Intermediate elevation: 30-100 μmol/L
Severe elevation: >100 μmol/L
The Pathophysiology: How Homocysteine Damages Vascular Health
Recent research has uncovered multiple mechanisms through which elevated homocysteine levels can contribute to vascular damage and cardiovascular disease. Understanding these pathways is essential for developing effective preventive and therapeutic strategies.
1. Oxidative Stress
Oxidative stress represents one of the primary mechanisms through which homocysteine exerts its deleterious effects on the cardiovascular system. Elevated homocysteine levels promote the generation of reactive oxygen species (ROS), leading to:
Increased oxidation of low-density lipoproteins (LDL)
Damage to vascular endothelial cells
Activation of pro-inflammatory signaling pathways
Reduction in nitric oxide bioavailability
2. Endothelial Dysfunction
The vascular endothelium, a single layer of cells lining blood vessels, plays a crucial role in maintaining vascular health. HHcy impairs endothelial function through:
Decreased nitric oxide (NO) production
Impaired endothelium-dependent vasodilation
Enhanced adhesion molecule expression
Altered endothelial cell proliferation and apoptosis
3. Inflammation
Chronic inflammation represents a key component in the development and progression of atherosclerosis. HHcy promotes vascular inflammation by:
Activating NF-κB and other pro-inflammatory transcription factors
Increasing production of inflammatory cytokines (IL-6, TNF-α)
Enhancing leukocyte recruitment and adhesion
Stimulating vascular smooth muscle cell (VSMC) proliferation
4. Epigenetic Modifications
Emerging research highlights the role of epigenetic changes in homocysteine-induced vascular damage. These include:
Altered DNA methylation patterns
Histone modifications
Regulation of microRNAs (miRNAs)
Changes in gene expression profiles
5. Lipoprotein Metabolism Disruption
HHcy can adversely affect lipoprotein metabolism, contributing to dyslipidemia and atherosclerosis through:
Enhanced LDL oxidation
Impaired HDL functionality
Altered lipoprotein(a) [Lp(a)] levels
Disrupted cholesterol efflux from foam cells
Recent Research Findings: The Evidence Connecting Homocysteine and Cardiovascular Disease
Multi-Ethnic Study of Atherosclerosis: Homocysteine and Heart Failure Risk
One groundbreaking study examined the relationship between total homocysteine (tHcy) levels and heart failure (HF) risk in the Multi-Ethnic Study of Atherosclerosis cohort, involving 6,765 participants. This research provided several important insights:
Elevated tHcy (>12 μmol/L) was significantly associated with overall heart failure risk
The association was particularly strong for heart failure with preserved ejection fraction (HFpEF)
The risk was amplified in individuals with dysglycemia (impaired fasting glucose or type 2 diabetes)
Homocysteine appeared to contribute more significantly to HFpEF risk than to heart failure with reduced ejection fraction (HFrEF)
Key Takeaway: This was the first study to demonstrate that homocysteine's impact differs by heart failure subtype, with stronger associations for HFpEF—a condition for which effective treatments remain limited.
Multicenter Study on Homocysteine and Coronary Syndromes
Another significant study including 381 coronary syndrome patients from Afghanistan, Egypt, and Pakistan examined the relationship between serum homocysteine levels and various types of coronary syndromes, as well as in-hospital mortality. The findings were striking:
A strong correlation existed between serum homocysteine levels and coronary syndrome severity (r = 0.4)
Significant differences in homocysteine levels were observed among different coronary syndrome groups
Each 1 μmol/L increase in homocysteine levels corresponded to a 10.5% increase in in-hospital mortality
Key Takeaway: This research establishes homocysteine not only as a valuable biomarker for coronary syndrome severity but also as a predictor of short-term mortality in these patients.
Systematic Review and Meta-Analysis on Global Variations
A comprehensive systematic review and meta-analysis assessed the association between homocysteine and coronary artery disease (CAD) across different geographic regions and time periods. After analyzing data from 59 studies, researchers found:
The pooled standardized mean difference (SMD) of homocysteine levels between CAD cases and controls was 0.73 (95% CI 0.55–0.91)
Asian populations showed the strongest association (SMD 0.85 [95% CI 0.60–1.10])
European populations demonstrated the weakest association (SMD 0.32 [95% CI 0.18–0.46])
The strength of association has been increasing over time (Beta = 0.0227, p = 0.048)
Key Takeaway: This meta-analysis highlights important geographic variations in the relationship between homocysteine and CAD, suggesting potential genetic, dietary, or environmental factors that may modify this association.
Managing Homocysteine Levels: Dietary and Lifestyle Approaches
Effective management of homocysteine levels involves a combination of dietary modifications, lifestyle changes, and in some cases, supplementation. Here's what the research suggests:
Dietary Interventions
1. Folate-Rich Foods
Folate (vitamin B9) plays a crucial role in homocysteine metabolism. Foods rich in folate include:
Leafy green vegetables (spinach, kale, broccoli)
Legumes (lentils, chickpeas, beans)
Fruits (oranges, bananas, melons)
Fortified cereals and grains
2. Vitamin B12 Sources
Vitamin B12 is essential for homocysteine remethylation. Good sources include:
Animal products (meat, fish, eggs)
Dairy products (milk, cheese, yogurt)
Fortified plant-based milks and cereals
This is particularly important for individuals following vegetarian or vegan diets, who may be at higher risk of B12 deficiency.
3. Vitamin B6-Rich Foods
Vitamin B6 contributes to homocysteine metabolism through the transsulfuration pathway. Sources include:
Meat (poultry, fish)
Whole grains
Bananas and other fruits
Vegetables (especially potatoes)
4. Mediterranean Diet
The Mediterranean diet, characterized by high consumption of fruits, vegetables, whole grains, olive oil, and moderate consumption of fish, has been linked to:
Improved homocysteine regulation
Reduced cardiovascular risk
Better overall vascular health
5. Omega-3 Fatty Acids and Magnesium
These nutrients have been shown to contribute to lowering homocysteine levels and mitigating its harmful vascular effects:
Omega-3 fatty acids: Found in fatty fish, flaxseeds, walnuts
Magnesium: Present in nuts, seeds, whole grains, leafy greens
Lifestyle Modifications
Several lifestyle factors significantly impact homocysteine levels and cardiovascular health:
1. Regular Physical Activity
Exercise has been shown to:
Lower homocysteine levels
Improve endothelial function
Reduce overall cardiovascular risk
2. Smoking Cessation
Smoking intensifies homocysteine elevation and vascular damage. Quitting smoking is a critical component of managing homocysteine-related cardiovascular risk.
3. Moderate Alcohol Consumption
Excessive alcohol consumption can elevate homocysteine levels. Limiting alcohol intake is advisable for individuals with or at risk for hyperhomocysteinemia.
The Supplementation Controversy: What the Clinical Trials Tell Us
Despite the established association between elevated homocysteine and cardiovascular disease, the effectiveness of B-vitamin supplementation in reducing cardiovascular events has yielded inconsistent results in clinical trials:
Major Clinical Trials
VISP (Vitamin Intervention for Stroke Prevention): Found no benefit of high-dose B vitamins on recurrent stroke risk
NORVIT (Norwegian Vitamin Trial): Showed no reduction in cardiovascular events with B-vitamin supplementation
HOPE-2 (Heart Outcomes Prevention Evaluation-2): Demonstrated modest stroke reduction but no effect on heart attack or cardiovascular death
Meta-Analyses
Recent meta-analyses have shown:
A modest but significant reduction in stroke risk with homocysteine-lowering interventions
No significant impact on coronary heart disease events
Possible greater benefits in populations without mandatory folate fortification
Why the Disconnect?
Several factors may explain the inconsistent results:
Genetic variability (e.g., MTHFR polymorphisms) affecting homocysteine metabolism
Different baseline homocysteine levels among study populations
Co-existing conditions such as hypertension, diabetes, and dyslipidemia
Timing of intervention (likely more effective as primary prevention)
Potential epigenetic changes that persist despite normalization of homocysteine levels
Emerging Therapeutic Approaches: Beyond B Vitamins
Recognizing the limitations of B-vitamin supplementation alone, researchers are exploring novel approaches to counteract homocysteine-induced vascular damage:
1. Antioxidant Therapies
Targeting oxidative stress through:
Direct antioxidants
Nrf2 activators
SOD mimetics
2. NADPH Oxidase Inhibitors
Inhibition of NADPH oxidase, a key enzyme in homocysteine-induced oxidative stress, represents a promising therapeutic strategy.
3. Epigenetic Modulators
Agents targeting DNA methylation patterns and histone modifications may help reverse homocysteine-induced epigenetic changes.
4. MicroRNA-Based Approaches
Targeting specific microRNAs implicated in homocysteine-mediated vascular damage, such as:
miR-217
miR-133
miR-143
5. Personalized Medicine Approaches
Tailoring interventions based on:
Genetic profiling (MTHFR genotype)
Baseline homocysteine levels
Co-existing cardiovascular risk factors
Epigenetic markers
Key Takeaways
Homocysteine is an independent risk factor for various cardiovascular conditions, including atherosclerosis, coronary artery disease, and heart failure.
Multiple mechanisms link elevated homocysteine to vascular damage, including oxidative stress, endothelial dysfunction, inflammation, and epigenetic changes.
The impact of homocysteine differs by heart failure subtype, with stronger associations for heart failure with preserved ejection fraction (HFpEF).
Each 1 μmol/L increase in homocysteine corresponds to approximately a 10.5% increase in in-hospital mortality in coronary syndrome patients.
Geographic variations exist in the strength of association between homocysteine and coronary artery disease, with Asian populations showing stronger associations than European populations.
Dietary and lifestyle interventions effectively lower homocysteine levels, particularly consumption of B-vitamin-rich foods and regular physical activity.
B-vitamin supplementation alone may be insufficient to reduce cardiovascular risk in all populations, suggesting the need for more comprehensive and personalized approaches.
Emerging therapies targeting oxidative stress, epigenetic modifications, and microRNAs show promise for mitigating homocysteine-induced vascular damage.
Frequently Asked Questions (FAQs)
Is a modestly elevated homocysteine level associated with an increased risk of cardiovascular disease?
Yes, research consistently shows that even moderately elevated homocysteine levels (>12 μmol/L) are significantly associated with increased cardiovascular risk, including coronary artery disease, stroke, and heart failure.
Is homocysteine a reliable predictor of cardiovascular disease (CVD)?
Homocysteine is considered an independent risk factor for CVD, with recent studies confirming its value as both a biomarker and predictor of cardiovascular events and mortality. However, it should be considered alongside traditional risk factors for comprehensive risk assessment.
Is homocysteine a risk factor for all types of vascular disease?
Homocysteine has been associated with various forms of vascular disease, including coronary artery disease, cerebrovascular disease, and peripheral arterial disease. Recent research suggests its impact may differ by disease subtype, with particularly strong associations for heart failure with preserved ejection fraction.
Does lowering homocysteine levels reduce the risk of cardiovascular disease?
The evidence is mixed. While B-vitamin supplementation effectively lowers homocysteine levels, large clinical trials have shown inconsistent results regarding cardiovascular outcomes. Meta-analyses suggest modest benefits for stroke prevention but limited impact on coronary heart disease events.
Is homocysteine a modifiable risk factor for cardiovascular disease?
Yes, homocysteine levels can be modified through dietary interventions (B-vitamin-rich foods), lifestyle changes (exercise, smoking cessation), and supplementation. However, the clinical benefit of these modifications may vary among individuals based on genetic factors, baseline homocysteine levels, and co-existing conditions.
How do I know if I have elevated homocysteine levels?
Homocysteine levels can be measured through a simple blood test. Discuss with your healthcare provider whether this test is appropriate for you, particularly if you have established cardiovascular disease, multiple risk factors, or a family history of premature cardiovascular events.
Call to Action
Understanding your cardiovascular risk profile is the first step toward better heart health. If you have established cardiovascular disease or multiple risk factors, talk to your healthcare provider about measuring your homocysteine levels and developing a personalized plan to manage them.
Remember that addressing homocysteine is just one aspect of comprehensive cardiovascular care. Focus on a heart-healthy lifestyle that includes a balanced diet rich in B vitamins, regular physical activity, smoking cessation, and management of traditional risk factors such as hypertension, diabetes, and dyslipidemia.
For healthcare professionals, consider incorporating homocysteine assessment into comprehensive cardiovascular risk evaluation, particularly for patients with unexplained cardiovascular disease, premature events, or those with resistant traditional risk factors.
As research continues to evolve, stay informed about emerging strategies for mitigating homocysteine-related cardiovascular risk and implementing evidence-based approaches in clinical practice.
Your heart health journey begins with awareness and action—take the first step today.
Related Articles
Can Type 2 Diabetes Be Reversed? The Critical Role of Beta Cells and Early Lifestyle Intervention
Mitochondrial Health and Exercise: What Every Athlete and Aging Adult Should Know | Healthnewstrend
Citations
Karger, A. B., Nomura, S. O., Guan, W., Garg, P. K., Tison, G. H., Szklo, M., Budoff, M. J., & Tsai, M. Y. (2025). Association Between Elevated Total Homocysteine and Heart Failure Risk in the Multi-Ethnic Study of Atherosclerosis Cohort. Journal of the American Heart Association, 14(5), e038168. https://doi.org/10.1161/JAHA.124.038168
Ullah, H., Huma, S., Naeem, L., Yasin, G., Ashraf, M., Tahir, N., Yunus, M., Shabana, H., Shalaby, A. H., Hassan Ali, A. A., (2025). Correlation of Serum Homocysteine Levels With Various Types of Coronary Syndromes (CS) and In-Hospital Mortality - A Multicenter Study. International journal of general medicine, 18, 725–732. https://doi.org/10.2147/IJGM.S500973
Unadkat, S. V., Padhi, B. K., Bhongir, A. V., Gandhi, A. P., Shamim, M. A., Dahiya, N., Satapathy, P., Rustagi, S., Khatib, (2024). Association between homocysteine and coronary artery disease—Trend over time and across the regions: A systematic review and meta-analysis. The Egyptian Heart Journal, 76(1), 1-16. https://doi.org/10.1186/s43044-024-00460-y
Tian, W., Ju, J., Guan, B., Wang, T., Zhang, J., Song, L., & Xu, H. (2025). Role of hyperhomocysteinemia in atherosclerosis: from bench to bedside. Annals of Medicine, 57(1). https://doi.org/10.1080/07853890.2025.2457527
Disclaimer
The information on this website is for informational purposes only and is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health care provider with any questions you may have regarding a medical condition or treatment. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
About the Author:
Dr.T.S. Didwal, MD, is an experienced Internal Medicine Physician with over 30 years of practice. Specializing in internal medicine, he is dedicated to promoting wellness, preventive health, and fitness as core components of patient care. Dr. Didwal’s approach emphasizes the importance of proactive health management, encouraging patients to adopt healthy lifestyles, focus on fitness, and prioritize preventive measures. His expertise includes early detection and treatment of diseases, with a particular focus on preventing chronic conditions before they develop. Through personalized care, he helps patients understand the importance of regular health screenings, proper nutrition, exercise, and stress management in maintaining overall well-being.
