TRT and Prostate Health: Is Testosterone Therapy Safe for Urinary Symptoms, Prostate and PSA?
Want to know if testosterone therapy is safe for your prostate? The TRAVERSE study reveals the latest findings on TRT and prostate health, including its impact on prostate cancer risk and urinary symptoms.
DR T S DIDWAL MD
8/28/20248 min read
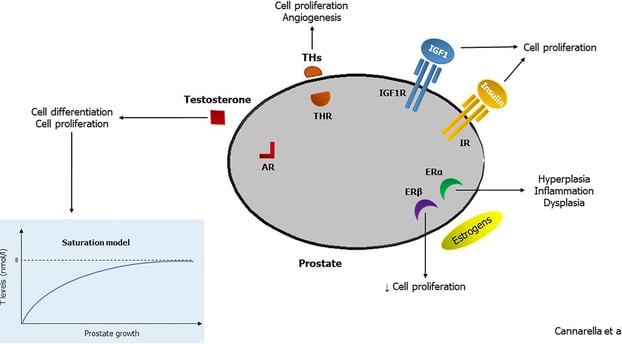

The TRAVERSE study has shown that testosterone replacement therapy (TRT) does not significantly increase the risk of prostate cancer or worsen urinary symptoms in men with low testosterone levels and no significant prostate issues. While there were slight increases in PSA levels, they were generally manageable. Overall, the study offers positive news for men considering TRT, but it's important to consult with a healthcare professional for personalized advice based on individual circumstances and risk factors.
Key Points
Low prostate cancer risk: TRT did not significantly increase the risk of prostate cancer in men with low baseline PSA levels.
Similar urinary symptoms: Both TRT and placebo groups experienced comparable urinary symptoms.
Modest PSA increases: PSA levels rose slightly in TRT recipients, but the increases were generally manageable.
Careful patient selection: Men with high PSA levels, severe urinary symptoms, or known prostate issues were excluded from the study.
Ongoing monitoring: Regular PSA monitoring and urological check-ups are recommended for men on TRT.
Shared decision-making: Patients and doctors should work together to decide if TRT is appropriate based on individual needs and risks.
New Insights on Testosterone Therapy and Prostate Health: Key Takeaways from the TRAVERSE Study
For years, the link between testosterone replacement therapy (TRT) and prostate health has stirred debate among medical professionals. Concerns about TRT potentially increasing prostate cancer risk or worsening urinary symptoms have led many doctors to approach this treatment cautiously. However, the groundbreaking TRAVERSE study published in JAMA Network Open offers the most comprehensive data to date, shedding new light on the relationship between TRT and prostate health.
This post will summarize the key findings of the TRAVERSE study and explore what they mean for men considering testosterone therapy. We’ll dive into the study’s methodology, its findings on prostate cancer risk and other related outcomes, and the broader implications for clinical practice.
Background on the TRAVERSE Study
The TRAVERSE study (Testosterone Replacement Therapy for Assessment of Long-Term Vascular Events and Efficacy Response in Hypogonadal Men) was a large-scale, randomized clinical trial aimed at evaluating the cardiovascular and prostate safety of TRT. This study was conducted in response to a 2015 FDA mandate requiring testosterone manufacturers to investigate the long-term effects of their products.
The study involved 5,204 men aged 45-80 with low testosterone levels (below 300 ng/dL) and symptoms of hypogonadism. To focus on a lower-risk population, men with high PSA levels (>3.0 ng/mL) or severe urinary symptoms were excluded.
Participants were randomly assigned to receive either testosterone gel or a placebo for an average of 22 months. Researchers closely monitored prostate-specific antigen (PSA) levels, performed regular prostate exams, and tracked outcomes such as prostate cancer diagnoses, urinary retention, and prostate surgeries.
Key Findings on Prostate Cancer Risk
One of the primary concerns with TRT is its potential to promote the growth of existing prostate cancers or increase the risk of developing new ones. The TRAVERSE study’s findings provide critical insights:
1. Low overall incidence of prostate cancer: Across more than 14,000 person-years of follow-up, only 23 cases of prostate cancer were diagnosed—12 in the testosterone group and 11 in the placebo group. This represents an incidence of less than 0.5% in both groups.
2. No significant difference in high-grade cancers: There were 5 cases of high-grade prostate cancer (Gleason score ≥4+3) in the testosterone group compared to 3 in the placebo group. The slight numerical difference was not statistically significant.
3. Similar rates across treatment arms: The hazard ratio for developing any prostate cancer was 1.07 (95% CI: 0.47-2.42) for testosterone vs. placebo, indicating no substantial increase in risk with TRT.
These findings suggest that in men with low baseline PSA levels, TRT does not significantly raise the short-to-medium term risk of prostate cancer compared to placebo.
Other Prostate-Related Outcomes
Beyond cancer risk, the TRAVERSE study examined several other prostate health measures:
1. Urinary symptoms: There was no significant difference between the groups in International Prostate Symptom Scores (IPSS), which assess the severity of lower urinary tract symptoms, indicating that TRT did not worsen urinary function.
2. Acute urinary retention: 20 men (0.77%) in the testosterone group and 16 men (0.61%) in the placebo group experienced acute urinary retention requiring catheterization, with no statistically significant difference between the groups.
3. Prostate surgeries: 23 men (0.89%) in the testosterone group underwent invasive prostate procedures, compared to 12 (0.46%) in the placebo group. Although numerically higher, this difference was not statistically significant (p=0.07).
4. New medications for urinary symptoms: The rates of starting new pharmacologic treatments for lower urinary tract symptoms were similar between groups (3.89% for testosterone vs. 3.34% for placebo).
Overall, these results suggest that TRT does not significantly worsen prostate-related symptoms or increase the need for procedures over the study’s approximately two-year follow-up period.
PSA Changes with Testosterone Therapy
As expected, men receiving testosterone experienced greater increases in PSA levels compared to the placebo group. Key observations included:
1. Modest overall increase: The average PSA increase attributable to testosterone was about 0.15 ng/mL at 12 months.
2. Plateauing effect: The difference in PSA levels between groups did not continue to widen after the first year of treatment.
3. Variation by baseline PSA: Men with higher baseline PSA levels (2–3 ng/mL) tended to experience larger increases than those with baseline levels below 1 ng/mL.
While PSA increases were observed, they were generally modest. The study employed careful monitoring protocols to determine when PSA increases warranted urologic referral.
Study Strengths and Limitations
The TRAVERSE study is the most rigorously conducted trial to date examining TRT and prostate outcomes. Key strengths include:
Large sample size of over 5,000 men
Randomized, placebo-controlled design
Careful screening to exclude high-risk men
Standardized monitoring protocols
Adjudicated prostate cancer outcomes
However, the study also had limitations:
Relatively short follow-up (~2 years on average) for cancer outcomes
Low overall number of prostate cancer events, limiting statistical power
Findings may not apply to men with higher baseline PSA levels or known prostate cancer
High rates of study discontinuation, though similar between groups
Implications for Clinical Practice
The TRAVERSE study has several important implications for testosterone therapy and prostate health:
1. Reassurance for lower-risk men: For men with low baseline PSA levels (<3 ng/mL) and no major risk factors, these data provide reassurance that short-to-medium term TRT is unlikely to significantly increase prostate cancer risk or worsen urinary symptoms.
2. Importance of careful patient selection: The study’s exclusion of men with high PSA levels, severe urinary symptoms, or known prostate nodules underscores the need for thorough evaluation before initiating TRT.
3. Value of standardized monitoring: The study’s structured protocol for PSA monitoring and urologic referrals could serve as a model for clinical practice, balancing cancer detection with avoiding unnecessary biopsies.
4. Shared decision-making: The study emphasized shared decision-making when PSA increases occurred, leading to relatively few biopsies while still detecting clinically significant cancers.
5. Ongoing vigilance: While the results are reassuring, they do not entirely eliminate concerns about long-term prostate risks with TRT. Continued monitoring remains essential, especially given the modest PSA increases observed.
Putting the Findings in Context
The TRAVERSE study’s findings align with other research on testosterone and prostate health:
Epidemiologic studies have generally found no consistent links between testosterone levels and prostate cancer risk.
Previous meta-analyses of smaller TRT trials also found no increased risk of prostate cancer, consistent with TRAVERSE.
Genetic studies have produced mixed results, with some suggesting a potential link between lifelong exposure to higher testosterone and prostate cancer risk.
The TRAVERSE results support the idea that TRT does not significantly alter prostate cancer risk in the short-to-medium term for appropriately selected patients. However, they do not rule out more subtle effects that could emerge over longer periods.
Unanswered Questions and Future Research
While TRAVERSE significantly advances our understanding, several important questions remain:
1. Long-term effects: What are the impacts of TRT on prostate outcomes beyond 2-3 years of treatment?
2. Higher-risk populations: Are the findings similar in men with higher baseline PSA levels or other risk factors?
3. Optimal monitoring: What is the most effective and cost-efficient way to monitor prostate health in men on long-term TRT?
4. Mechanisms: If TRT does not increase prostate cancer risk, what are the biological mechanisms that explain this?
5. Other testosterone formulations: Do the findings apply equally to other forms of TRT beyond topical gels?
Future studies addressing these questions will help refine the approach to testosterone therapy and prostate health.
Conclusion
The TRAVERSE study represents a significant step forward in understanding how testosterone replacement therapy affects prostate health. In a large population of carefully selected men with hypogonadism, TRT was not associated with a meaningful increase in prostate cancer diagnoses, severe urinary symptoms, or the need for prostate procedures over an average follow-up of two years.
These findings should help alleviate concerns that have made doctors hesitant to prescribe TRT, even to men who could benefit. However, they also underscore the importance of thorough evaluation before starting therapy and ongoing monitoring during treatment.
The decision to use testosterone therapy should be made on an individual basis, weighing the potential benefits against the risks. The TRAVERSE results provide valuable new data to inform those decisions, allowing for a more evidence-based approach to managing hypogonadism in aging men.
For patients considering TRT, these findings highlight the importance of:
1. Thorough initial evaluation, including PSA testing and assessment of urinary symptoms
2. Choosing a doctor experienced in managing testosterone therapy
3. Adhering to recommended monitoring protocols
4. Open communication about any new symptoms or concerns during treatment
By following these principles and staying informed about the latest research, patients and doctors can work together to maximize the benefits of testosterone therapy while minimizing potential risks to prostate health.
Faqs
Question 1: What is the primary concern regarding testosterone replacement therapy (TRT) and prostate health?
Answer: The primary concern is that TRT might increase the risk of prostate cancer or worsen urinary symptoms.
Question 2: What was the main goal of the TRAVERSE study?
Answer: The TRAVERSE study aimed to evaluate the cardiovascular and prostate safety of TRT.
Question 3: What were the key findings of the TRAVERSE study regarding prostate cancer risk?
Answer: The study found no significant increase in prostate cancer risk among men with low baseline PSA levels who received TRT.
Question 4: Did TRT worsen urinary symptoms in the TRAVERSE study?
Answer: No, TRT did not significantly worsen urinary symptoms in the study participants.
Question 5: How did TRT affect PSA levels?
Answer: TRT led to modest increases in PSA levels, but these increases were generally manageable.
Question 6: What are the implications of the TRAVERSE study for clinical practice?
Answer: The study provides reassurance for men with low PSA levels considering TRT. However, careful patient selection and ongoing monitoring remain crucial.
Question 7: What are the limitations of the TRAVERSE study?
Answer: The study had a relatively short follow-up period, and its findings may not apply to all men, especially those with higher baseline PSA levels or other risk factors.
Journal Reference
Bhasin, S., Travison, T. G., Pencina, K. M., O’Leary, M., Cunningham, G. R., Lincoff, A. M., Nissen, S. E., Lucia, M. S., Preston, M. A., Khera, M., Khan, N., Snabes, M. C., Li, X., Tangen, C. M., Buhr, K. A., & Thompson, I. M. (2023). Prostate Safety Events During Testosterone Replacement Therapy in Men With Hypogonadism. JAMA Network Open, 6(12), e2348692. https://doi.org/10.1001/jamanetworkopen.2023.48692
Image Credit:https://www.frontiersin.org/files/Articles/554078/fendo-12-554078-HTML-r1/image_m/fendo-12-554078-g001.jpg
Related
https://healthnewstrend.com/testosterone-deficiency-in-older-men-can-trt-help
Disclaimer
The information on this website is for educational and informational purposes only and is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or treatment, and before undertaking a new healthcare regimen, and never disregard professional medical advice or delay in seeking it because of something you have read on this website.
