How Obesity Triggers Chronic Inflammation: The Immuno-Metabolic Nexus Explained
Discover how obesity drives chronic low-grade inflammation through the complex interplay between immune and metabolic pathways. This evidence-based review explores key cytokines, adipokines, and cellular mechanisms linking adipose tissue to systemic inflammation.
DR T S DIDWAL MD
4/17/20259 min read
The Link Between Inflammation and Obesity: A Comprehensive Review
Obesity represents one of the most significant public health challenges worldwide, with implications far beyond excess weight.
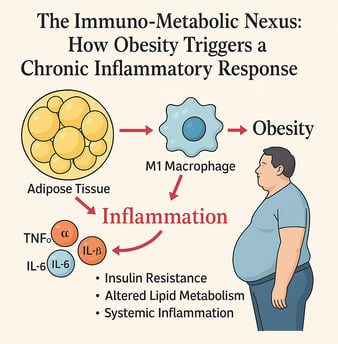
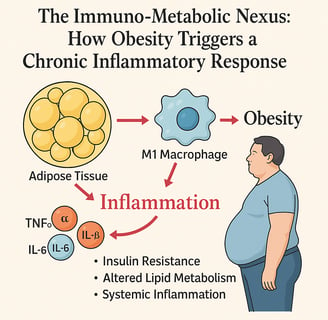
Obesity is more than excess fat—it’s a state of chronic low-grade inflammation that disrupts both immune and metabolic balance. This review explores the immuno-metabolic nexus, where adipose tissue, once thought to be a passive storage depot, actively secretes pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β. These molecules promote insulin resistance, alter lipid metabolism, and trigger systemic inflammation that underlies many obesity-related diseases, including type 2 diabetes, cardiovascular disease, and even cancer.
The shift in immune cell populations within fat—especially the dominance of M1 macrophages—fuels this inflammatory state. Additionally, adipokines like leptin and adiponectin modulate inflammatory responses, with leptin promoting inflammation and adiponectin offering protective, anti-inflammatory effects—though paradoxically decreased in obesity.
Emerging research also highlights the impact of diet and lifestyle on these pathways, with pro-inflammatory diets elevating C-reactive protein (CRP) and other biomarkers. Understanding this intricate interplay opens new doors for therapeutic strategies targeting inflammation—not just weight loss.
By addressing the inflammatory component of obesity, we can develop more effective, holistic approaches to managing metabolic health. It’s not just about the fat—it’s about what that fat is doing behind the scenes.
This post explores the latest scientific understanding of inflammation in obesity, examining key cytokines, adipokines, and their role in metabolic homeostasis.
The Inflammatory Nature of Obesity
Obesity is not merely an energy storage problem but rather a complex condition characterized by chronic low-grade inflammation. The adipose tissue, once considered simply a passive repository for excess calories, is now recognized as an active endocrine organ capable of producing various bioactive substances, including pro-inflammatory cytokines.
According to recent research, elevated levels of inflammatory cytokines including TNF-α, IL-6, IL-1β, and MCP-1 play pivotal roles in the development of this low-grade inflammation in individuals with obesity. These cytokines contribute significantly to dyslipidemia and insulin resistance, setting the stage for metabolic dysfunction.
Key Inflammatory Mediators in Obesity
Interleukin-6 (IL-6)
IL-6 is a multifunctional cytokine produced by immune cells, adipocytes, vascular cells, and other cell types. Its biology is influenced by complex signaling pathways, particularly those mediated by JAK/STAT. When bound to its receptor, IL-6 initiates signaling cascades that activate diverse cellular responses related to inflammation, immune regulation, and metabolism.
In obesity, IL-6 levels are often elevated, contributing to the chronic inflammatory state. Studies show that IL-6 plays a dual role – it can promote inflammation but also has some anti-inflammatory properties depending on the context.
Tumor Necrosis Factor-α (TNF-α)
TNF-α represents one of the most well-studied cytokines in the context of obesity-related inflammation. This pro-inflammatory cytokine is significantly upregulated in adipose tissue of obese individuals and contributes to:
Impaired insulin signaling
Enhanced lipolysis
Increased production of other pro-inflammatory cytokines
Recruitment of inflammatory cells to adipose tissue
These effects collectively worsen metabolic dysfunction and insulin resistance.
Adipose Tissue as an Inflammatory Hub
Adipose tissue undergoes significant changes in obesity, becoming a site of chronic low-grade inflammation. Beyond its role as an energy storage depot, adipose tissue functions as an active endocrine organ secreting various bioactive substances, including inflammatory cytokines.
A primary driver of inflammatory conditions in adipose tissue is the infiltration of immune cells, particularly macrophages. In lean individuals, adipose tissue contains predominantly M2 macrophages with anti-inflammatory properties. However, in obesity, there's a shift toward M1 macrophages that produce pro-inflammatory cytokines, exacerbating the inflammatory state.
The Complex Interplay Within Adipose Tissue
The relationship among adipocytes, immune cells, and resident macrophages within the adipose tissue microenvironment is intricate and dynamic. This interaction is essential for maintaining metabolic homeostasis and regulating immune responses.
In obesity, adipocytes undergo hypertrophy (enlargement) and hyperplasia (increased number), leading to:
Hypoxia due to inadequate blood supply
Cellular stress and death
Release of pro-inflammatory mediators
Recruitment and activation of immune cells
This cascade of events perpetuates and amplifies the inflammatory response, creating a vicious cycle that contributes to systemic inflammation and metabolic dysfunction.
Adipokines: Mediators of Inflammation and Metabolism
Adipokines play a crucial role in the delicate interaction between adipose tissue, inflammation, and metabolism. Two important adipokines significantly impacting these processes are adiponectin and leptin.
Adiponectin
Primarily released by adipocytes, adiponectin regulates glucose levels and fatty acid breakdown. It possesses anti-inflammatory properties that help mitigate inflammatory responses. Interestingly, despite being produced by adipose tissue, adiponectin levels are paradoxically decreased in obesity, contributing to the pro-inflammatory state and insulin resistance.
Leptin
Often referred to as the "satiety hormone," leptin signals to the brain about energy stores. In obesity, despite high leptin levels (hyperleptinemia), individuals develop leptin resistance, where the brain becomes less responsive to leptin's appetite-suppressing effects. Beyond its role in appetite regulation, leptin also influences immune function and inflammation, with high levels potentially promoting pro-inflammatory responses.
Inflammation, Obesity, and Cancer Risk
The link between obesity and cancer risk is increasingly recognized, with chronic low-grade inflammation playing a central role in this relationship. Adipose tissue in obese individuals acts as a dynamic endocrine organ, releasing pro-inflammatory cytokines and creating a widespread inflammatory state.
This chronic inflammation, combined with metabolic disruptions, creates an environment conducive to:
DNA damage
Cell proliferation
Angiogenesis
Inhibition of apoptosis
All these factors contribute to increased cancer risk across multiple organ systems.
The Dietary Connection to Inflammation in Obesity
Recent studies highlight the significant impact of dietary patterns on inflammatory markers in individuals with obesity. Research using the Dietary Inflammatory Index (DII) has established correlations between high DII scores and increased levels of inflammation markers.
Pro-inflammatory Diets
Diets rich in processed foods, refined carbohydrates, and saturated fats tend to promote inflammation. Studies consistently show that pro-inflammatory diets are associated with elevated levels of inflammatory markers such as C-reactive protein (CRP) and interleukin-6 (IL-6) in individuals with obesity.
A recent study examining 124 people with obesity found that higher DII scores were significantly associated with elevated CRP levels (p = 0.006), indicating that the inflammatory potential of diet directly impacts systemic inflammation markers. Moreover, as the inflammatory burden of diet increased, Body Mass Index (BMI) also increased (p = 0.009), suggesting a direct relationship between inflammatory dietary patterns and obesity severity.
Anti-inflammatory Diets
Conversely, anti-inflammatory diets rich in fiber, omega-3 fatty acids, and polyphenols while low in processed foods and refined sugars may help reduce inflammatory markers. However, the extent of these effects varies across studies, populations, and methodologies, highlighting the heterogeneity of findings in this area.
Systemic Immune Inflammation Index (SII) in Pediatric Obesity
Recent research has explored the relationship between the Systemic Immune Inflammation Index (SII) and central obesity in children, a particularly vulnerable population. Using waist-to-height ratio (WHtR), subcutaneous fat, and visceral fat as obesity proxies, researchers have found compelling associations between SII and central obesity.
In a study of 4,730 individuals, adolescents in the highest quartile of SII levels exhibited the greatest risk for central obesity (OR=3.07, 95% CI:2.45-3.87) compared to those in the lowest quartile. Longitudinal data from 1,425 subjects further revealed that individuals in the highest SII quartile had the highest risk of developing central obesity (RR=1.83, 95% CI:1.18-2.83) over time.
These findings suggest that SII could serve as a valuable predictor of central obesity in the pediatric population, potentially enabling earlier intervention strategies to prevent obesity-related complications.
Severe Obesity, Inflammation, and Mortality Risk
Severe obesity (BMI ≥ 35 kg/m²) is associated with inflammation and insulin resistance (IR), which may increase mortality and cancer risks. A comprehensive study involving 163,008 participants from the UK Biobank cohort investigated these relationships and examined whether lifestyle factors could modify these associations.
The research found that severe obesity, inflammation, and IR were each independently associated with increased risks of all-cause mortality [HRs(95%CIs) 1.24(1.17–1.30), 1.63(1.55–1.72), and 1.11(1.05–1.17)] and all-site cancers [1.06(1.02–1.10), 1.14(1.10–1.19), and 1.02(0.99–1.06)].
Even more significantly, joint analyses revealed elevated risks due to interaction between severe obesity, inflammation, and IR, with the highest HRs(95%CIs) of 1.88(1.67–2.11) for all-cause mortality and 1.20(1.08–1.34) for all-site cancers.
Importantly, the study demonstrated that favorable lifestyles could attenuate these risks, highlighting the potential for lifestyle modifications to mitigate the adverse health outcomes associated with obesity-related inflammation.
Targeting Inflammatory Cytokines for Obesity-Related Comorbidities
The therapeutic potential of addressing inflammatory cytokines in relation to obesity comorbidities is substantial. Pro-inflammatory cytokines like TNF-alpha, IL-6, and IL-1β are enhanced in obesity-related chronic low-grade inflammatory states and implicated in the development of insulin resistance, dyslipidemia, endothelial dysfunction, and other metabolic disorders.
Emerging therapeutic approaches include:
Pharmacological interventions targeting specific cytokines or their receptors
Nutritional strategies focusing on anti-inflammatory dietary components
Lifestyle modifications including physical activity programs designed to reduce inflammation
Bariatric surgery, which has been shown to reduce inflammatory markers in addition to promoting weight loss
Key Takeaways
Obesity represents a state of chronic low-grade inflammation characterized by elevated levels of pro-inflammatory cytokines including TNF-α, IL-6, IL-1β, and MCP-1.
Adipose tissue functions as an active endocrine organ, producing various bioactive substances that regulate inflammatory signaling pathways and metabolic homeostasis.
The interplay between immune cells, macrophages, and adipocytes exacerbates inflammatory processes in obese individuals, further aggravating metabolic dysfunction.
Diet significantly impacts inflammatory markers in obesity, with pro-inflammatory diets consistently associated with elevated inflammatory biomarkers.
The Systemic Immune Inflammation Index (SII) shows promise as a predictor of central obesity, particularly in pediatric populations.
Severe obesity interacts with inflammation and insulin resistance to increase the risks of mortality and cancer, but these risks can be mitigated by healthy lifestyle choices.
Targeting inflammatory cytokines and adipokines offers promising opportunities for developing novel therapies for obesity-related complications.
FAQs
Does dietary intake influence the development of obesity through inflammatory pathways?
Yes, dietary patterns significantly influence inflammatory pathways. Pro-inflammatory diets rich in processed foods and saturated fats promote inflammation and contribute to obesity development, while anti-inflammatory diets rich in fiber, polyphenols, and omega-3 fatty acids may help reduce inflammation and associated metabolic dysfunction.
Does dietary intake affect inflammatory index scores in people with obesity?
Research demonstrates that dietary choices directly impact inflammatory index scores in obese individuals. Studies using the Dietary Inflammatory Index (DII) show that consumption of pro-inflammatory foods correlates with higher inflammatory markers like CRP, while anti-inflammatory foods help reduce these markers.
Are obesity and inflammation associated with a high risk of cancer?
Yes, obesity-related chronic inflammation creates an environment conducive to cancer development by promoting DNA damage, abnormal cell proliferation, and inhibiting apoptosis. The relationship between obesity, inflammation, and cancer involves complex interactions among adipokines, cytokines, and metabolic dysregulation.
Does diet affect inflammatory markers and obesity?
Diet significantly affects both inflammatory markers and obesity. Research shows that higher Dietary Inflammatory Index scores correlate with elevated CRP levels and increased BMI, highlighting the direct relationship between dietary patterns, inflammation, and obesity severity.
Are obesity and inflammation associated with increased risk of all-cause mortality?
Evidence from large cohort studies confirms that obesity, particularly severe obesity, combined with inflammation and insulin resistance, significantly increases the risk of all-cause mortality. However, adherence to healthy lifestyle practices can substantially mitigate these elevated risks.
Can inflammatory cytokines be used to treat comorbidities associated with obesity?
Targeting inflammatory cytokines represents a promising therapeutic approach for obesity-related comorbidities. Emerging strategies include pharmacological interventions against specific cytokines, anti-inflammatory dietary approaches, physical activity programs, and surgical interventions that collectively address the inflammatory component of obesity.
Call to Action
Understanding the intricate relationship between inflammation and obesity offers new avenues for prevention and treatment strategies. If you're struggling with obesity or related health issues, consider these evidence-based approaches:
Evaluate your diet for inflammatory potential using tools like the Dietary Inflammatory Index
Incorporate anti-inflammatory foods such as fatty fish, leafy greens, nuts, olive oil, and berries
Discuss inflammatory markers with your healthcare provider as part of your comprehensive health assessment
Adopt an active lifestyle that includes regular physical activity to help reduce systemic inflammation
Consider the whole picture – addressing inflammation through multiple lifestyle approaches rather than focusing solely on weight loss
By addressing the inflammatory component of obesity, we can develop more effective strategies for preventing and managing this complex condition and its associated comorbidities. The emerging research highlighted in this review underscores the importance of a comprehensive approach that considers not just weight management but also the underlying inflammatory processes that contribute to obesity-related health risks.
Related Articles
Is HIIT Better Than Cardio for Fat Loss? A Scientific Deep Dive | Healthnewstrend
Obesity and Cardiovascular Disease: Can Antioxidant Balance Reduce Your Risk? | Healthnewstrend
Citations
Isidoro, B.M., Beretta, M.V., Flores, P.T. et al. Inflammation Diet and the Association with Inflammatory Markers in Individuals with Obesity - A Systematic Scoping Review. Curr Nutr Rep 14, 60 (2025). https://doi.org/10.1007/s13668-025-00653-0
Zhang, Q., Kong, B., Zhou, Z., Liu, F., Wen, E., Lin, B., Xuan, P., Lu, W., Su, Z., Li, Y., Tang, Y., Xiong, J., Yao, P., & Li, Y. (2025). Association between systemic immune-inflammation index and central obesity in pediatric populations: A cross-sectional and cohort study. Frontiers in Immunology, 16, 1546612. https://doi.org/10.3389/fimmu.2025.1546612
Uti, D. E., Atangwho, I. J., Omang, W. A., Alum, E. U., Obeten, U. N., Udeozor, P. A., Agada, S. A., Bawa, I., & Ogbu, C. O. (2025). Cytokines as key players in obesity low grade inflammation and related complications. Obesity Medicine, 54, 100585. https://doi.org/10.1016/j.obmed.2025.100585
Jin, Q., Liu, S., Zhang, Y., Ji, Y., Wu, J., Duan, H., Liu, X., Li, J., Zhang, Y., Lyu, Z., Song, F., Song, F., Li, H., & Huang, Y. (2025). Severe obesity, high inflammation, insulin resistance with risks of all-cause mortality and all-site cancers, and potential modification by healthy lifestyles. Scientific Reports, 15(1), 1-11. https://doi.org/10.1038/s41598-025-85519-9
Toğuç, H., Öngün Yılmaz, H., & Yaprak, B. (2025). Exploring the link between dietary inflammatory index, inflammatory biomarkers, and sleep quality in adults with obesity: A pilot investigation. International Journal of Obesity, 1-6. https://doi.org/10.1038/s41366-025-01728-2
Disclaimer
The information on this website is for educational and informational purposes only, and is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or treatment, and before undertaking a new healthcare regimen, and never disregard professional medical advice or delay in seeking it because of something you have read on this website.
About the Author:
Dr.T.S. Didwal, MD, is an experienced Internal Medicine Physician with over 30 years of practice. Specializing in internal medicine, he is dedicated to promoting wellness, preventive health, and fitness as core components of patient care. Dr. Didwal’s approach emphasizes the importance of proactive health management, encouraging patients to adopt healthy lifestyles, focus on fitness, and prioritize preventive measures. His expertise includes early detection and treatment of diseases, with a particular focus on preventing chronic conditions before they develop. Through personalized care, he helps patients understand the importance of regular health screenings, proper nutrition, exercise, and stress management in maintaining overall well-being.
