Remarkable Breakthrough: Nasal Spray Shows Promise in Clearing Alzheimer's-Related Tau Proteins
Discover a groundbreaking new approach to treating Alzheimer's disease. Learn how an innovative nasal spray targets and clears toxic tau proteins, offering hope for millions affected by this devastating condition.
DR T S DIDWAL MD
8/12/20248 min read
Researchers have made a significant breakthrough in Alzheimer's disease treatment with the development of a novel nasal spray therapy that targets toxic tau proteins. According to the research published in Science Translational Medicine, this innovative approach uses a specialized antibody, TTCM2, which selectively binds to and clears pathological tau aggregates, crucial in neurodegenerative disorders like Alzheimer's. Unlike traditional treatments, TTCM2 is encapsulated in lipid micelles, enabling it to cross the blood-brain barrier and reach the brain, a significant challenge in neurological therapies. The nasal spray demonstrated promising results in mouse models, effectively clearing toxic tau, improving cognitive function, and restoring synaptic proteins. This suggests its potential as a non-invasive and patient-friendly treatment option. The TTCM2 antibody works by engaging the TRIM21 protein, which tags the harmful tau-antibody complex for destruction, helping to clear toxic tau from brain cells.
Key Points
Nasal Spray Therapy: Researchers have developed a nasal spray using a specialized antibody (TTCM2) to clear toxic tau proteins, which are linked to Alzheimer's disease and other tauopathies.
Targeted Treatment: The TTCM2 antibody selectively binds to and clears harmful tau aggregates, leaving healthy tau proteins unaffected, offering a targeted approach to treating neurodegenerative diseases.
Innovative Drug Delivery: The TTCM2 antibody is encapsulated in lipid micelles, allowing it to cross the blood-brain barrier and reach the brain, a significant challenge in treating neurological disorders.
Promising Mouse Model Results: In mice with Alzheimer's-like symptoms, the nasal spray effectively cleared pathological tau, improved cognitive function, and restored synaptic proteins, suggesting its potential effectiveness.
TRIM21 Protein Mechanism: The TTCM2 antibody’s effectiveness relies on the TRIM21 protein, which tags the tau-antibody complex for destruction by the cell’s proteasome, clearing toxic tau aggregates from brain cells.
Non-Invasive Administration: The nasal spray offers a non-invasive, potentially patient-friendly method of treatment, that could be self-administered at home if proven effective in humans.
Future Implications: While still in early research stages, this therapy could lead to major breakthroughs in Alzheimer's treatment, pending successful human trials and further research on its long-term effects and applicability to other tauopathies.
A Breath of Fresh Air in Alzheimer's Research: Nasal Spray Shows Promise in Clearing Toxic Tau Proteins
In the relentless battle against Alzheimer's disease and other neurodegenerative disorders, researchers have long sought effective ways to target and clear the toxic proteins that accumulate in patients' brains. A groundbreaking study published in the prestigious journal Science Translational Medicine offers a glimmer of hope with a novel approach: a nasal spray that can effectively clear toxic tau proteins from the brains of mice with Alzheimer's-like symptoms. This innovative therapy, developed by a multi-institutional team of neuroscientists, represents a significant step forward in the field of tauopathy research. By combining a specially designed antibody with advanced drug delivery techniques, the researchers have created a treatment that could potentially revolutionize how we approach Alzheimer's disease and related disorders.
Understanding Tauopathies:
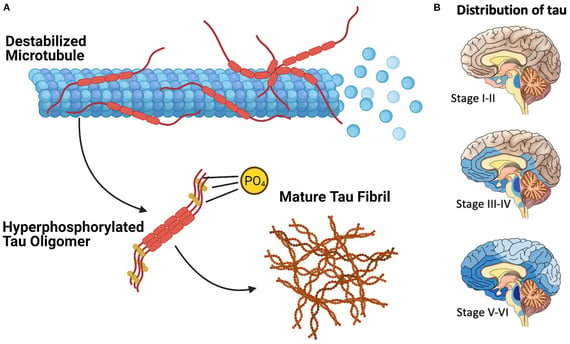
Before delving into the details of this exciting new research, it's crucial to understand the role of tau proteins in neurodegenerative diseases. Tau is a structural protein that plays a vital role in maintaining the internal structure of neurons, the brain's primary information-processing cells. In healthy brains, tau proteins help stabilize microtubules, which are essential for transporting nutrients and other important molecules within neurons. However, in various neurodegenerative disorders collectively known as tauopathies, tau proteins become abnormally modified and begin to aggregate into toxic clumps inside neurons. These aggregates, often referred to as "tau tangles," disrupt normal cellular functions and contribute to the progressive death of brain cells. Alzheimer's disease is the most well-known tauopathy, but this group of disorders also includes frontotemporal dementia, progressive supranuclear palsy, and other conditions.
The Challenge of Targeting Tau:
One of the major hurdles in developing effective treatments for tauopathies has been the difficulty of selectively targeting and clearing these toxic tau aggregates. Many of these aggregates form inside neurons, making them challenging to reach with traditional drug therapies. Additionally, it's crucial to target only the abnormal, toxic forms of tau while leaving healthy tau proteins unaffected. Previous attempts at tau immunotherapy—using antibodies to target and clear tau proteins—have shown limited success in clinical trials. These approaches have struggled to effectively clear intracellular tau aggregates and improve cognitive function in patients.
A New Approach: The TTCM2 Antibody:
The research team behind this new study took a novel approach to overcome these challenges. They developed a specialized antibody called toxic tau conformation-specific monoclonal antibody-2, or TTCM2 for short. What makes TTCM2 unique is its ability to selectively recognize and bind to pathological tau aggregates – the harmful clumps of tau protein that contribute to neurodegeneration. The researchers demonstrated that TTCM2 could effectively recognize pathological tau aggregates in brain tissue samples from patients with various tauopathies, including Alzheimer's disease, dementia with Lewy bodies, and progressive supranuclear palsy. This selectivity is crucial, as it allows the antibody to target only the harmful forms of tau while leaving normal, functional tau proteins untouched.
Moreover, TTCM2 showed a remarkable ability to inhibit tau-seeding activity. Tau seeding refers to the process by which abnormal tau proteins can induce normal tau to misfold and aggregate, spreading the pathology throughout the brain. By interfering with this process, TTCM2 has the potential to slow or halt the progression of tauopathies.
Overcoming the Blood-Brain Barrier:
While developing an effective antibody was a significant achievement, the researchers faced another major challenge: how to deliver TTCM2 to the brain. The blood-brain barrier, a protective membrane that separates the brain's blood vessels from the surrounding tissue, prevents many substances, including most antibodies, from entering the brain from the bloodstream. To overcome this obstacle, the research team employed an innovative approach. They encapsulated TTCM2 in specially designed lipid micelles—tiny bubble-like structures made of fat molecules. These micelles serve as a protective vehicle for the antibodies, allowing them to pass through the blood-brain barrier and reach their targets within the brain.
The Intranasal Delivery System:
Perhaps the most exciting aspect of this new therapy is its method of administration. Rather than relying on invasive injections or oral medications that might struggle to reach the brain, the researchers opted for an intranasal delivery system. By formulating the TTCM2-loaded micelles (TTCM2-ms) into a nasal spray, they created a non-invasive and potentially more effective way to deliver the treatment directly to the brain.
This intranasal approach offers several advantages:
1. Rapid delivery: The nasal passage provides a direct route to the brain, allowing for quicker uptake of the medication.
2. Bypassing the blood-brain barrier: While the micelles help the antibodies cross the blood-brain barrier, the intranasal route may allow some of the medication to bypass this barrier entirely, entering the brain through the olfactory and trigeminal nerve pathways.
3. Non-invasive administration: A nasal spray is much easier and more comfortable for patients to use compared to injections or other more invasive delivery methods.
4. Potential for home use: If proven safe and effective in humans, this type of therapy could potentially be self-administered by patients at home, improving treatment adherence and quality of life.
Promising Results :
To test the effectiveness of their novel therapy, the researchers conducted a series of experiments using mice genetically engineered to develop tau pathology similar to that seen in human Alzheimer's disease. The results were striking. A single intranasal dose of TTCM2-ms effectively cleared pathological tau from the brains of these mice. Moreover, the treatment led to an increase in synaptic proteins—molecules essential for communication between neurons – and significantly improved cognitive function in aged tauopathy mice. These findings suggest that the TTCM2-ms nasal spray not only clears toxic tau aggregates but also has the potential to reverse some of the damaging effects of tau pathology on brain function.
Unraveling the Mechanism: The Role of TRIM21:
To better understand how TTCM2-ms achieves its effects, the researchers delved into the underlying mechanisms. Their investigations revealed a crucial player in the process: a protein called tripartite motif-containing 21, or TRIM21. TRIM21 is an intracellular antibody receptor and E3 ubiquitin ligase – in simpler terms, it's a protein that can recognize antibodies inside cells and tag other proteins for destruction by the cell's waste disposal system, the proteasome. The researchers found that TRIM21 was essential for TTCM2-ms to clear intracellular tau aggregates effectively.
This discovery sheds light on how the therapy works at a molecular level:
1. The TTCM2 antibodies, protected by their micelle shells, enter brain cells.
2. Inside the cells, TTCM2 binds to pathological tau aggregates.
3. TRIM21 recognizes the antibody-bound tau complexes.
4. TRIM21 tags these complexes for destruction by the proteasome.
5. The cell's proteasome system breaks down the toxic tau aggregates, effectively clearing them from the cell.
This mechanism allows TTCM2-ms to target not only extracellular tau aggregates but also those hidden inside neurons, a significant advantage over previous tau immunotherapy approaches.
Implications for Future Alzheimer's Treatments:
While these results are undoubtedly exciting, it's important to note that this research is still in its early stages. The therapy has shown promising results in mouse models and human tissue samples, but it has not yet been tested in human patients. Extensive clinical trials will be necessary to determine if this approach is safe and effective for humans with Alzheimer's disease or other tauopathies.
However, if this nasal spray therapy proves successful in human trials, it could represent a major breakthrough in the treatment of Alzheimer's disease and related disorders. Some potential advantages of this approach include:
1. Targeted treatment: By specifically targeting pathological tau aggregates, this therapy could potentially slow or halt disease progression with fewer side effects than less selective treatments.
2. Non-invasive administration: The nasal spray delivery method could make treatment more accessible and less burdensome for patients.
3. Potential for early intervention: If proven safe, this type of therapy could potentially be used in the early stages of disease or even as a preventive measure in high-risk individuals.
4. Combination therapy potential: This approach could potentially be combined with other treatments targeting different aspects of Alzheimer's pathology, such as amyloid-beta plaques, for a more comprehensive treatment strategy.
Challenges and Future Directions:
While the results of this study are highly encouraging, several challenges and questions remain to be addressed as research moves forward:
1. Human trials: The safety and efficacy of this therapy need to be thoroughly evaluated in human patients. This will involve extensive clinical trials, which can take several years to complete.
2. Long-term effects: The current study focused on the effects of a single dose of TTCM2-ms. Future research will need to investigate the long-term effects of repeated dosing and determine the optimal treatment regimen.
3. Applicability to different tauopathies: While the therapy showed promise in Alzheimer's disease models, its effectiveness in other tauopathies needs to be further explored.
4. Manufacturing and scalability: Producing antibodies and formulating them into micelles for nasal delivery on a large scale may present technical and economic challenges that need to be addressed.
5. Combination with other therapies: Investigating how this treatment might work in combination with other Alzheimer's therapies could lead to more effective treatment strategies.
Conclusion:
The development of this novel nasal spray therapy for clearing toxic tau proteins represents a significant advance in the field of Alzheimer's research. By combining a highly specific antibody with innovative drug delivery techniques, the researchers have created a potential treatment that addresses many of the challenges that have hindered previous tau immunotherapy attempts. While much work remains to be done before this therapy could become available to patients, the results of this study offer new hope in the fight against Alzheimer's disease and other tauopathies. As research continues, we may be one step closer to effective treatments that can slow, halt, or even reverse the progression of these devastating neurodegenerative disorders.
As we look to the future, it's clear that innovative approaches like this one, which combine advances in immunology, neuroscience, and drug delivery, will be crucial in developing the next generation of treatments for Alzheimer's disease and related disorders. While the road ahead may be long, each breakthrough brings us closer to a world where neurodegenerative diseases no longer rob individuals of their memories, their independence, and their lives.
Journal Reference:
Meftah, S., Durrant, C. S., & Spires-Jones, T. L. (2024). A nose for tau. Science Translational Medicine, 16(754). https://doi.org/10.1126/scitranslmed.adq6489becomes
Image credit: https://www.frontiersin.org/files/Articles/671458/fmolb-08-671458-HTML/image_m/fmolb-08-671458-g002.jpg
Related
https://healthnewstrend.com/how-does-obesity-impact-brain-function-and-increase-dementia-risk
Disclaimer
The information on this website is for informational purposes only and is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or treatment. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
