Shift Work, Sleep Loss, and Diabetes: Is Your Circadian Clock at Risk?
Discover how shift work and sleep deprivation can disrupt your circadian rhythm and increase your risk of developing type 2 diabetes. Learn about the connection between your body's internal clock and your health. Find tips for improving sleep and managing diabetes.
DR T S DIDWAL MD
8/7/20249 min read
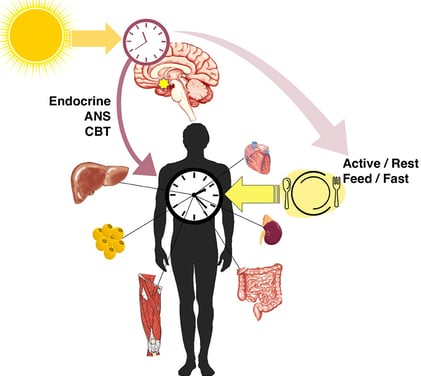
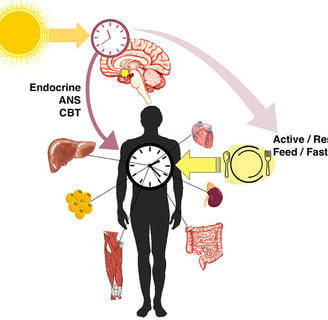
This review published in Frontiers in Physiology explores the intricate link between the circadian clock, our body's internal timekeeper, and type 2 diabetes (T2D). Disruptions to circadian rhythms, often caused by shift work, irregular sleep patterns, or genetic factors, can contribute to the development and progression of T2D. The circadian clock influences various metabolic processes, including insulin secretion, glucose metabolism, and lipid metabolism. When disrupted, these processes can lead to insulin resistance, hyperglycemia, and ultimately T2D. The blog also discusses the role of the HPA axis, which is influenced by the circadian clock and plays a key role in glucose regulation. Potential therapeutic approaches targeting the circadian clock, such as cryptochrome stabilizers, metformin, and natural compounds, are explored. While research is still ongoing, understanding this connection offers promising avenues for developing new and effective treatments for T2D.
Key Points
Circadian Rhythm Disruption: Irregular sleep patterns, shift work, and jet lag can disrupt the body's internal clock, increasing the risk of developing type 2 diabetes (T2D).
Insulin Regulation: The circadian clock controls the timing of insulin secretion. Disruptions can lead to impaired insulin production and release, contributing to T2D.
Glucose Metabolism: The circadian clock regulates how the body processes glucose. Disruptions can lead to insulin resistance and difficulty controlling blood sugar levels.
HPA Axis Involvement: The circadian clock influences the HPA axis, which regulates stress hormones. Chronic stress can contribute to T2D, and the circadian clock plays a role in this connection.
Potential Therapeutic Targets: Understanding the circadian clock's role in T2D has opened up new avenues for treatment, including medications that target specific clock genes.
Lifestyle Importance: Maintaining a regular sleep schedule, avoiding excessive artificial light, and managing stress are crucial for supporting healthy circadian rhythms and reducing T2D risk.
The Circadian Clock and Type 2 Diabetes: Unraveling the Complex Connection
Type 2 diabetes (T2D) is a chronic metabolic disorder that affects millions of people worldwide. As its prevalence continues to rise, researchers are exploring new avenues to understand its underlying mechanisms and develop innovative treatments. One area of growing interest is the relationship between T2D and the circadian clock – our body's internal timekeeping system. This blog post delves into the fascinating connection between circadian rhythms and T2D, exploring how disruptions in our biological clock may contribute to the development and progression of this disease.
Understanding Type 2 Diabetes
Before we dive into the circadian connection, let's briefly review what T2D is and how it develops. T2D is characterized by elevated blood glucose levels resulting from two main issues:
Insulin resistance: The body's cells become less responsive to insulin, the hormone responsible for facilitating glucose uptake.
Pancreatic β-cell dysfunction: The insulin-producing cells in the pancreas gradually lose their ability to secrete sufficient insulin to maintain normal blood glucose levels.
In the early stages of T2D, the pancreas tries to compensate for insulin resistance by producing more insulin. However, over time, this overproduction leads to β-cell burnout, resulting in insufficient insulin secretion and the onset of full-blown T2D.
The Circadian Clock: Our Internal Timekeeper
The circadian clock is a complex biological system that regulates various physiological processes in a roughly 24-hour cycle. This internal timekeeping mechanism helps organisms adapt to the daily light-dark cycle and coordinates numerous bodily functions, including metabolism, hormone secretion, and sleep-wake patterns.
At the molecular level, the circadian clock operates through a transcription-translation feedback loop involving several key genes and proteins. The primary players in this loop include:
BMAL1 and CLOCK: These proteins form a complex that activates the transcription of other clock genes.
PER and CRY: These proteins accumulate over time and eventually inhibit the activity of BMAL1 and CLOCK, creating a negative feedback loop.
ROR and REV-ERB: These proteins fine-tune the expression of BMAL1, adding another layer of regulation to the clock mechanism.
The Circadian Clock-Diabetes Connection
Growing evidence suggests that disruptions in circadian rhythms may contribute to the development of T2D. Here are some key findings that highlight this connection:
Shift work and increased T2D risk: Studies have shown that people who work night shifts or rotating shifts have a higher risk of developing T2D. This may be due to the misalignment between their work schedules and natural circadian rhythms.
Sleep duration and T2D: Both short (less than 6 hours) and long (more than 9 hours) sleep durations have been associated with an increased risk of T2D compared to the optimal 7-8 hours of sleep per night.
Genetic links: Variations in circadian clock genes, such as BMAL1, CLOCK, and CRY1, have been associated with an increased risk of T2D in various populations.
Animal studies: Mice with mutations in clock genes often exhibit symptoms similar to T2D, including hyperglycemia, insulin resistance, and impaired glucose tolerance.
Molecular Mechanisms Linking Circadian Rhythms and T2D
The circadian clock influences T2D development through several interconnected mechanisms:
Regulation of insulin secretion: The circadian clock controls the rhythmic secretion of insulin from pancreatic β-cells. Disruptions in clock genes can lead to impaired insulin production and release.
Insulin sensitivity: The responsiveness of tissues to insulin follows a circadian pattern. Clock gene mutations can result in increased insulin resistance in muscle, liver, and adipose tissue.
Glucose metabolism: The circadian clock regulates the expression of genes involved in glucose uptake, glycogen synthesis, and gluconeogenesis in the liver and muscle tissues.
Lipid metabolism: Adipose tissue exhibits rhythmic expression of clock genes, which control lipid metabolism. Disruption of these rhythms can lead to dyslipidemia and contribute to insulin resistance.
Hypothalamic-Pituitary-Adrenal (HPA) axis regulation: The circadian clock influences the HPA axis, which controls the production of glucocorticoids like cortisol. These hormones play a crucial role in glucose homeostasis, and their dysregulation can contribute to T2D progression.
The HPA Axis: A Key Player in the Circadian-Diabetes Connection
The HPA axis is a complex neuroendocrine system that plays a vital role in maintaining homeostasis, including glucose regulation. Here's how the circadian clock interacts with the HPA axis to influence T2D risk:
Rhythmic hormone secretion: The circadian clock regulates the rhythmic release of corticotropin-releasing hormone (CRH) from the hypothalamus and adrenocorticotropic hormone (ACTH) from the pituitary gland. These hormones ultimately control the production and release of glucocorticoids from the adrenal glands.
Glucocorticoid effects: Glucocorticoids, such as cortisol, have wide-ranging effects on metabolism. They can increase blood glucose levels by promoting gluconeogenesis in the liver and reducing insulin sensitivity in peripheral tissues.
Bidirectional relationship: While the circadian clock regulates the HPA axis, glucocorticoids can also feedback on the clock system, creating a complex interplay between these two regulatory mechanisms.
HPA axis dysfunction in T2D: Disruption of the HPA axis can lead to chronically elevated glucocorticoid levels, contributing to insulin resistance, hyperglycemia, and β-cell dysfunction – all hallmarks of T2D.
Circadian misalignment: Shift work, jet lag, and other forms of circadian disruption can lead to inappropriate activation of the HPA axis, potentially exacerbating T2D risk or progression.
Therapeutic Approaches Targeting the Circadian Clock
Understanding the relationship between the circadian clock and T2D has opened up new avenues for therapeutic interventions. Here are some promising approaches:
Cryptochrome stabilizers: Compounds like TW68 and KL001 stabilize CRY proteins, key components of the circadian clock. These stabilizers have shown potential in reducing blood glucose levels and suppressing gluconeogenesis in animal models.
Metformin: This widely used T2D medication has been found to upregulate BMAL1 expression, suggesting that part of its therapeutic effect may be due to circadian clock modulation.
Nobiletin: This natural compound found in citrus peels has shown promise in improving insulin sensitivity and lowering blood glucose levels by upregulating various clock genes.
Casein kinase inhibitors: Compounds like PF-5006739 inhibit casein kinase 1δ and ε, which are involved in regulating clock protein stability. These inhibitors have shown potential in normalizing disrupted circadian gene expression and improving glucose tolerance.
Thiazolidinediones: These insulin-sensitizing drugs have been found to resolve disruptions in clock gene expression, potentially contributing to their therapeutic effects.
Psychiatric medications: Some antidepressants, antipsychotics, and mood stabilizers that regulate clock gene expression have been associated with improved glycemic control in T2D patients.
Future Directions and Challenges
While the link between circadian rhythms and T2D is becoming increasingly clear, there are still many questions to be answered and challenges to overcome:
Translation to humans: Much of the current evidence comes from animal studies. More research is needed to fully understand how these findings translate to human physiology and pathology.
Personalized chronotherapy: Given the complexity of circadian rhythms and individual variations, developing personalized approaches to circadian-based T2D treatments will be crucial.
Timing of interventions: The efficacy of treatments targeting the circadian clock may depend on the timing of administration. Determining the optimal timing for different interventions will be an important area of research.
Long-term effects: As circadian rhythms regulate numerous physiological processes, it's essential to carefully evaluate the long-term effects of circadian-targeted therapies on overall health.
Combination therapies: Exploring how circadian-based treatments can be combined with existing T2D therapies to enhance efficacy and reduce side effects will be an important area of investigation.
Lifestyle interventions: Developing evidence-based recommendations for sleep, meal timing, and light exposure to support healthy circadian rhythms and reduce T2D risk.
High-throughput screening: Utilizing advanced screening techniques to identify novel compounds that can modulate specific circadian clock components for T2D treatment.
Conclusion
The intricate relationship between the circadian clock and Type 2 Diabetes reveals a fascinating area of research with significant potential for improving our understanding and treatment of this widespread metabolic disorder. By recognizing the importance of circadian rhythms in glucose homeostasis and insulin signaling, we open up new possibilities for innovative therapeutic approaches.
As research in this field progresses, we may see the development of more targeted and effective treatments for T2D that leverage our body's natural timekeeping mechanisms. Moreover, this knowledge emphasizes the importance of maintaining healthy circadian rhythms through lifestyle choices, potentially offering new strategies for T2D prevention.
While there is still much to learn about the complex interplay between circadian rhythms, metabolism, and diabetes, the growing body of evidence suggests that paying attention to our internal clocks may be a crucial factor in managing and preventing T2D. As we continue to unravel
Faqs:
1. What is the circadian clock, and how does it work?
Answer: The circadian clock is an internal timekeeping system that regulates various physiological processes in a roughly 24-hour cycle, aligning them with the day-night cycle. It operates through a transcription-translation feedback loop involving key genes and proteins like BMAL1, CLOCK, PER, and CRY. These components interact in a coordinated manner to regulate the timing of biological functions such as hormone secretion, metabolism, and sleep-wake patterns.
2. How does the circadian clock affect metabolism?
Answer: The circadian clock plays a crucial role in regulating metabolism by controlling the timing of various metabolic processes. It influences insulin secretion, insulin sensitivity, glucose uptake, glycogen synthesis, and gluconeogenesis. Disruptions in circadian rhythms can lead to metabolic imbalances, contributing to conditions like type 2 diabetes by impairing insulin action and glucose metabolism.
3. Can disrupted sleep patterns increase the risk of Type 2 Diabetes?
Answer: Yes, disrupted sleep patterns can increase the risk of type 2 diabetes. Both insufficient sleep (less than 6 hours) and excessive sleep (more than 9 hours) have been associated with a higher risk of developing T2D. Irregular sleep schedules, such as those experienced by shift workers, can misalign the circadian clock, leading to metabolic disturbances and an increased risk of diabetes.
4. What is the connection between shift work and type 2 diabetes?
Answer: Shift work, particularly night shifts and rotating shifts, can disrupt the natural circadian rhythms by misaligning the internal clock with external cues like light and dark. This misalignment can lead to impaired insulin sensitivity, disrupted glucose metabolism, and increased levels of cortisol, all of which contribute to a higher risk of developing type 2 diabetes.
5. How do genetic variations in circadian clock genes influence the risk of type 2 diabetes?
Answer: Genetic variations in circadian clock genes, such as BMAL1, CLOCK, and CRY1, can affect the functioning of the circadian system. These variations can lead to altered rhythms in hormone secretion, metabolism, and insulin sensitivity, increasing the risk of developing Type 2 Diabetes. Studies have identified specific polymorphisms in these genes that are associated with an elevated risk of T2D in different populations.
6. What role does the Hypothalamic-Pituitary-Adrenal (HPA) axis play in the relationship between circadian rhythms and Type 2 Diabetes?
Answer: The HPA axis, regulated by the circadian clock, controls the release of glucocorticoids like cortisol, which are crucial for glucose homeostasis. The circadian clock regulates the rhythmic secretion of CRH and ACTH, influencing cortisol levels. Disruptions in the HPA axis can lead to chronically elevated glucocorticoid levels, contributing to insulin resistance and hyperglycemia, which are key features of Type 2 Diabetes.
7. Are there therapeutic approaches targeting the circadian clock to treat Type 2 Diabetes?
Answer: Yes, several therapeutic approaches target the circadian clock to treat Type 2 Diabetes. These include:
Cryptochrome stabilizers: Compounds like TW68 and KL001 stabilize CRY proteins, reducing blood glucose levels.
Metformin: This medication upregulates BMAL1 expression, suggesting a circadian modulation effect.
Nobiletin: A natural compound that improves insulin sensitivity by upregulating clock genes.
Casein kinase inhibitors: Compounds like PF-5006739 normalize disrupted circadian gene expression.
Thiazolidinediones: Insulin-sensitizing drugs that also resolve clock gene disruptions.
Psychiatric medications: Some antidepressants and antipsychotics that regulate clock gene expression have been linked to improved glycemic control.
8. How can lifestyle changes support healthy circadian rhythms and reduce the risk of type 2 diabetes?
Answer: Lifestyle changes that support healthy circadian rhythms can reduce the risk of Type 2 Diabetes. These include maintaining a consistent sleep schedule, ensuring adequate sleep duration (7-8 hours), timing meals appropriately, managing light exposure (especially reducing blue light at night), and incorporating regular physical activity. These practices help align the circadian clock with environmental cues, promoting better metabolic health and reducing diabetes risk.
Journal Reference
Tran, H. T., Kondo, T., Ashry, A., Fu, Y., Okawa, H., Sawangmake, C., & Egusa, H. (2024). Effect of circadian clock disruption on type 2 diabetes. Frontiers in Physiology, 15, 1435848. https://doi.org/10.3389/fphys.2024.1435848
Image credit:https://www.frontiersin.org/files/Articles/519741/fnut-07-00039-HTML/image_m/fnut-07-00039-g002.jpg
Related
https://healthnewstrend.com/how-do-carbohydrates-affect-blood-sugar-control-in-diabetics
https://healthnewstrend.com/how-metabolic-syndrome-and-insulin-resistance-impact-diabetes-treatment
Disclaimer
The information on this website is for informational purposes only and is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or treatment. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
